Abstract
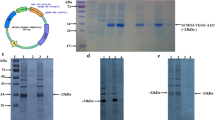
Endostatin, a 20 kDa C-terminal fragment of collagen XVIII, is a specific inhibitor of endothelial cell proliferation and angiogenesis. In the present study, we produced soluble and biologically active recombinant human endostatin (rhEndostatin) in Escherichia coli by expressing via fusion with solubility-promoting peptides and optimizing the expression conditions. The rhEndostatin was expressed via fusion with glutathione S-transferase (GST) and NusA protein, respectively. It revealed that NusA protein enhanced the production of soluble rhEndostatin; but GST didn’t. By optimizing the expression conditions, the production of soluble NusA-rhEndostatin fusion protein was about 50% of total cellular proteins and about 90% of the products appeared in the cellular supernatant fraction. The soluble NusA-rhEndostatin fusion protein was purified by one-step hydrophobic interaction chromatography and NusA was removed by thrombin. Then rhEndostatin was purified by affinity chromatography and gel filtration chromatography. As a result, a simple and economical purification procedure for rhEndostatin isolation was obtained. The biological activity of the rhEndostatin was demonstrated in vitro using a human vascular endothelial cells (HuVECs) proliferation assay. Our study provides a feasible and convenient approach to produce soluble and biologically active rhEndostatin.
Similar content being viewed by others
References
O’Reilly, M. S., T. Boehm, and J. Folkman (1997) Endostatin: An endogenous inhibitor of angiogenesis and tumor growth. Cell 88: 277–285.
Boehm, T., M. S. O’Reilly, K. Keough, J. Shiloach, R. Shapiro, and J. Folkman (1998) Zinc-binding of endostatin is essential for its antiangiogenic activity. Biochem. Biophys. Res. Commun. 252: 190–194.
Ding, Y. H., K. Javaherian, K. M. Lo, R. Chopra, T. Boehm, J. Lanciotti, B. A. Harris, Y. Li, R. Shapiro, E. Hohenester, R. Timpl, J. Folkman, and D. C. Wiley (1998) Zinc-dependent dimmers observed in crystals of human endostatin. Proc. Natl. Acad. Sci. USA 95: 10443–10448.
Sasaki, T., N. Fukai, K. Mann, W. Göhring, B. R. Olsen, and R. Timpl (1998) Structure, function and tissue forms of the Cterminal globular domain of collagen XVIII containing the angiogenesis inhibitor endostatin. EMBO J. 17: 4249–4256.
Boehm, T., J. Folkman, and M. S. O’Reilly (1997) Antiangiogenic therapy of experimental cancer does not induce acquired drug resistance. Nature 390: 404–407.
Sudhakar, A., H. Sugimoto, and C. Q. Yang (2003) Human tumstatin and human endostatin exhibit distinct antiangiogenic activities mediated by αVβ3 and α5β1 integrins. Proc. Natl. Acad. Sci. USA 100: 4766–4771.
Chura-Chambi, R. M., P. H. Tornieri, P. J. Spencer, P. A. Nascimento, M. B. Mathor, and L. Morganti (2004) High-level synthesis of recombinant murine endostatin in Chinese hamster ovary cells. Protein Expr. Purif. 35: 11–16.
Dhanabal, M., R. Volk, R. Ramchandran, M. Simons, and V. P. Sukhatme (1999) Cloning, expression, and in vitro activity of human endostatin. Biochem. Biophys. Res Comm. 258: 345–352.
Violand, B. N. and E. I. Harding (1999) Method of producing mouse and human endostatin. US Patent 6,653,098.
Xu, H. M., G. Y. Zhang, X. D. Ji, L. Cao, L. Shu, and Z. C. Hua (2005) Expression of soluble, biologically active recombinant human endostatin in Escherichia coli. Protein Expr. Purif. 41: 252–258.
You, W. K., S. H. So, L. Hyosil, S. Y. Park, M. R. Yoon, S. I. Chang, H. K. Kim, Y. A. Joe, Y. K. Hong, and S. I. Chung (1999) Purification and characterization of recombinant murine endostatin in Escherichia coli. Exp. Mol. Med. 31: 197–202.
Bradford, M. M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248–254.
Sun, Z., W. Lu, Y. Tang, J. Zhang, J. Chen, H. Deng, X. Li, and J. N. Liu (2007) Expression, purification, and characterization of human urodilatin in Escherichia coli. Protein Expr. Purif. 55: 312–318.
Wohlkönig, A., M. Sénéchal, F. Dewitte, K. Backers, C. Erneux, and V. Villeret (2007) Expression and purification in high yield of a functionally active recombinant human Type I inositol (1,4,5)P3 5-phosphatase. Protein Expr. Purif. 55: 69–74.
Yan, B., J. T. Zhou, J. Wang, C. H. Du, H. M. Hou, Z. Y. Song, and Y. M. Bao (2004) Expression and characteristics of the gene encoding azoreductase from Rhodobacter sphaeroides AS1.1737. FEMS Microbiol. Lett. 236: 129–136.
Niiranen, L., S. Espelid, C. R. Karlsen, M. Mustonen, S. M. Paulsen, P. Heikinheimo, and N. P. Willassen (2007) Comparative expression study to increase the solubility of cold adapted Vibrio proteins in Escherichia coli. Protein Expr. Purif. 52: 210–218.
Davis, G. D., C. Elisee, D. M. Newham, and R. G. Harrison (1999) New fusion protein systems designed to give soluble expression in Escherichia coli. Biotechnol. Bioeng. 65: 382–388.
Bessette, P. H., F. Aslund, J. Beckwith, and G. Georgiou (1999) Efficient folding of proteins with multiple disulfide bonds in the Escherichia coli cytoplasm. Proc. Natl. Acad. Sci. USA 96: 13703–13708.
Dhanabal, M., R. Ramchandran, R. Volk, I. E. Stillman, M. Lombardo, M. L. Iruela-Arispe, M. Simons, and V. P. Sukhatme (1999) Endostatin: Yeast production. Mutants and antitumor effect in renal cell carcinoma. Cancer Res. 59: 189–197.
Hohenester, E., T. Sasaki, R. B. Olsen, and R. Timpl (1998) Crystal structure of the angiogenesis inhibitor endostatin at 1.5 A resolution. EMBO J. 17: 1656–1664.
Miosge, N., T. Sasaki, and R. Timpl (1999) Angiogenesis inhibitor endostatin is a distinct component of elastic fibers in vessel walls. FASEB J. 13: 1743–1750.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Du, C., Yi, X. & Zhang, Y. Expression and purification of soluble recombinant Human Endostatin in Escherichia coli . Biotechnol Bioproc E 15, 229–235 (2010). https://doi.org/10.1007/s12257-009-0100-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-009-0100-5