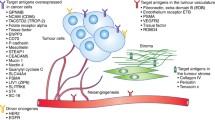
Abstract
Chemotherapy and targeted agents, such as small molecules and monoclonal antibodies, have individually improved cancer medical therapeutics, yet there is still an unmet need for resistant or refractory disease. Drug combinations are usually more effective, but dose-limiting toxicities are of concern. The idea of developing a “smart bomb” treatment dates many years back. Nowadays, the development of biotechnology and the deeper knowledge of molecular biology have made possible the engineering of tumor-specific antibodies bearing cytotoxins directly and solely targeting the tumor cell, thus minimizing toxicity and increasing efficacy. Approved agents include trastuzumab emtansine (T-DM1) for breast cancer, brentuximab vedotin for Hodgkins’ disease, and gemtuzumab ozogamacin for relapsed acute myeloid leukemia. The present short review presents several newer antibody–drug conjugates (ADCs) in clinical studies and discusses ways to optimize ADC future design.
Similar content being viewed by others
References
Neuberg DS. Reprise: gemtuzumab ozogamicin for older patients with acute myeloid leukemia. J Clin Oncol. 2012;30:3905–6.
Okeley NM, et al. Intracellular activation of SGN-35, a potent anti-CD30 antibody-drug conjugate. Clin Cancer Res. 2010;16:888–97.
Boyraz B, et al. Trastuzumab emtansine for HER2-positive breast cancer. Curr Med Res Opin. 2013 Mar 1;29(4):405–14.
Gorovits B, Krinos-Fiorotti C. Proposed mechanism of off-target toxicity for antibody-drug conjugates driven by mannose receptor uptake. Cancer Immunol Immunother. 2013 Feb;62:217–23.
Petersdorf S, et al. A phase 3 study of gemtuzumab ozogamicin during induction and postconsolidation therapy in younger patients with acute myeloid leukemia. Blood. 2013;121(24):4854–60.
Oflazoglu E, et al. Potent anticarcinoma activity of the humanized anti-CD70 antibody h1F6 conjugated to the tubulin inhibitor auristatin via an uncleavable linker. Clin Cancer Res. 2008;14:6171–80.
Mathur R, Weiner G. Picking the optimal target for antibody-drug conjugates. Alexandria: ASCO Educational book;2013.
Vlachakis D, Kossida S. Antibody drug conjugate bioinformatics: drug delivery through the letterbox. Comput Math Methods Med. 2013;2013:282398.
Alley SC, et al. Contribution of linker stability to the activities of anticancer immunoconjugates. Bioconjug Chem. 2008;19(3):759–65.
Flygare J, Pillow T, Aristoff P. Antibody-drug conjugates for the treatment of cancer. Chem Biol Drug Des. 2013;81:113–21.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tryfonopoulos, D. Antibody–cytotoxic drug conjugates in clinical trials and future perspectives: the development of Trojan horses. memo 7, 47–49 (2014). https://doi.org/10.1007/s12254-013-0124-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12254-013-0124-6