Abstract
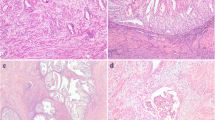
The aim of this study was to examine the associations among the haphazard invasive patterns, defined as directionless infiltration into the myometrium; expression of key proteins; tumor infiltrative lymphocytes (TILs); and the prognosis of gade-3 endometrioid carcinoma (G3EC). Between 1990 and 2013, patients with G3EC who underwent surgery at our hospital were identified. Invasive patterns were classified into either haphazard, infiltrative, or expansile patterns. The estrogen, progesterone, androgen receptor, cytokeratin 5/6, epidermal growth factor receptor, E-cadherin, snail-2, vimentin, ZEB1, chromogranin A, synaptophysin, MLH1, MSH2, MSH6, and PMS2 levels were evaluated by immunochemical analysis. The degree of strong or weak lymphocyte infiltration (LI) were evaluated using zone formation of LI at the invasive front. Haphazard, infiltrative, and expansile patterns were discovered in 8 (18%), 6 (13%), and 31 (69%) cases, respectively. Cases with the haphazard patterns were diagnosed at a more advanced stage (p < 0.01) and recurred more frequently (p < 0.01). There were statistical differences in progression-free survival (PFS) and overall survival (OS) between the three groups (PFS; p < 0.01: OS; p < 0.01). In multivariate analysis, only the haphazard pattern was found to be an independent, worse prognostic factor of PFS (Hazard ratio (HR) =10.8, p < 0.01) and OS (HR = 23.3, p < 0.01). Furthermore, the haphazard invasive pattern was related with weak LI (p < 0.01) but not with the expression of all proteins analyzed. The haphazard pattern was found to be a worse prognostic factor and was associated with weak LI in G3EC. The aggressive feature of G3EC might be associated with LI but not tumor biology.






Similar content being viewed by others
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Prat J (2015) Pathology of cancers of the female genital tract. Int J Gynaecol Obstet 131(Suppl 2):S132–S145. https://doi.org/10.1016/j.ijgo.2015.06.010.
Cancer Genome Atlas Research Network, Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H, Robertson AG, Pashtan I, Shen R, Benz CC, Yau C, Laird PW, Ding L, Zhang W, Mills GB, Kucherlapati R, Mardis ER, Levine DA (2013) Integrated genomic characterization of endometrial carcinoma. Nature 497:67–73. https://doi.org/10.1038/nature12113
Clinical Practice Guideline in Oncology. National Comprehensive Cancer Network. Http://www.nccn.org/professionals/physician-gls/PDF/uterine.pdf. Accessed 04 Jan 2019
Morice P, Leary A, Creutzberg C, Abu-Rustum N, Darai E (2016) Endometrial cancer. Lancet. 387:1094–1108. https://doi.org/10.1016/S0140-6736(15)00130-0 Epub 2015 Sep 6
Kurman RJ, Carcangiu ML, Herrington CS, Young RH (2014) WHO classification of tumours of female reproductive organs. International Agency for Research on Cancer, Lyon, pp 126–133
Lewin SN, Herzog TJ, Barrena Medel NI, Deutsch I, Burke WM, Sun X, Wright JD (2010) Comparative performance of the 2009 international federation of gynecology and obstetrics' staging system for uterine corpus cancer. Obstet Gynecol 116:1141–1149. https://doi.org/10.1097/AOG.0b013e3181f39849
Wang J, Jia N, Li Q, Wang C, Tao X, Hua K, Feng W (2016) Analysis of recurrence and survival rates in grade 3 endometrioid endometrial carcinoma. Oncol Lett 12:2860–2867. https://doi.org/10.3892/ol.2016.4918
Rasool N, Fader AN, Seamon L, Neubauer NL, Shahin FA, Alexander HA, Moore K, Moxley K, Secord AA, Kunos C, Rose PG, O'Malley DM (2010) Stage I, grade 3 endometrioid adenocarcinoma of the endometrium: an analysis of clinical outcomes and patterns of recurrence. Gynecol Oncol 116:10–14. https://doi.org/10.1016/j.ygyno.2009.10.043 Epub 2009 Oct 28
Miyamoto M, Takano M, Tsuda H, Matuura H, Aoyama T, Soyama H, Kato K, Iwahashi H, Ishibashi H, Yoshikawa T, Suzuki A, Hirata J, Furuya K (2018) Zone formation of lymphocyte infiltration at invasive front as a biomarker of prognosis in endometrial carcinomas. Oncology 3:1–8. https://doi.org/10.1159/000493010
McIntyre JB, Nelson GS, Ghatage P, Morris D, Duggan MA, Lee CH, Doll CM, Köbel M (2014) PIK3CA missense mutation is associated with unfavorable outcome in grade 3 endometrioid carcinoma but not in serous endometrial carcinoma. Gynecol Oncol 132:188–193. https://doi.org/10.1016/j.ygyno.2013.11.015
Meng B, Hoang LN, McIntyre JB, Duggan MA, Nelson GS, Lee CH, Köbel M (2014) POLE exonuclease domain mutation predicts long progression-free survival in grade 3 endometrioid carcinoma of the endometrium. Gynecol Oncol 132:188–193. https://doi.org/10.1016/j.ygyno.2014.05.006.
Suzuki C, Matsumoto T, Sonoue H, Arakawa A, Furugen Y, Kinoshita K (2003) Prognostic significance of the infiltrative pattern invasion in endometrioid adenocarcinoma of the endometrium. Pathol Int 53:495–500
Longacre TA, Hendrickson MR (1999) Diffusely infiltrative endometrial adenocarcinoma. An adenoma malignum pattern of myoinvasion. Am J Surg Pathol 23:69–78
Iwatsuki M, Mimori K, Yokobori T, Ishi H, Beppu T, Nakamori S, Baba H, Mori M (2010) Epithelial-mesenchymal transition in cancer development and its clinical significance. Cancer Sci 101:293–299. https://doi.org/10.1111/j.1349-7006.2009.01419.x
Colas E, Pedrola N, Devis L, Ertekin T, Campoy I, Martínez E, Llauradó M, Rigau M, Olivan M, Garcia M, Cabrera S, Gil-Moreno A, Xercavins J, Castellvi J, Garcia A, Ramon y Cajal S, Moreno-Bueno G, Dolcet X, Alameda F, Palacios J, Prat J, Doll A, Matias-Guiu X, Abal M, Reventos J (2012) The EMT signaling pathways in endometrial carcinoma. Clin Transl Oncol 14:715–720. https://doi.org/10.1007/s12094-012-0866-3
Eichhorn JH, Young RH (2001) Neuroendocrine tumors of the genital tract. Am J Clin Pathol 115(Suppl):S94–S112. https://doi.org/10.1309/64CW-WKGK-49EF-BYD1
van Hoeven KH, Hudock JA, Woodruff JM, Suhrland MJ (1995) Small cell neuroendocrine carcinoma of the endometrium. Int J Gynecol Pathol 14:21–29
Niikura H, Sasano H, Matsunaga G, Watanabe K, Ito K, Sato S, Yajima A (1995) Prognostic value of epidermal growth factor receptor expression in endometrioid endometrial carcinoma. Hum Pathol 26:892–896
Stefansson IM, Salvesen HB, Akslen LA (2006) Loss of p63 and cytokeratin 5/6 expression is associated with more aggressive tumors in endometrial carcinoma patients. Int J Cancer 118:1227–1233. https://doi.org/10.1002/ijc.21415
Piulats JM, Matias-Guiu X (2016) Immunotherapy in endometrial Cancer: in the Nick of time. Clin Cancer Res 22:5623–5625. https://doi.org/10.1158/1078-0432.CCR-16-1820
Alvarez T, Miller E, Duska L, Oliva E (2012) Molecular profile of grade 3 endometrioid endometrial carcinoma: is it a type I or type II endometrial carcinoma? Am J Surg Pathol 36:753–761. https://doi.org/10.1097/PAS.0b013e318247b7bb
Acknowledgements
We would like to thank Editage (www.editage.jp) for English language editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Research Involving Human Participants and/or Animals
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required. This study was approved by the Ethics Committee of the National Defense Medical College, Tokorozawa, Japan.
Informed Consent
Because our study was retrospective study, informed consent was not obtained.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Miyamoto, M., Takano, M., Tsuda, H. et al. The Haphazard Pattern in Grade-3 Endometrioid Carcinoma Is Associated with Poor Prognosis and Tumor Lymphocyte Infiltration. Pathol. Oncol. Res. 26, 783–790 (2020). https://doi.org/10.1007/s12253-019-00624-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-019-00624-1