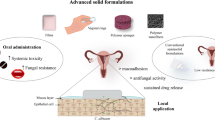
Abstract
Purpose
The purpose of this study was to develop fluconazole microsponges loaded bioadhesive film, rolled into a torpedo-shaped capsule, to achieve ease of application and reduce application frequency for the treatment of vulvovaginal candidiasis. This was accomplished by using biodegradable polymer blend specifically designed to cater the busy lifestyle of working women.
Methods
Microsponges were prepared using the quasi-emulsion solvent diffusion method. Herein, fluconazole: ethyl cellulose (1:2.5, 1:3, 1:3.5) and polyvinyl alcohol (used as the external phase) were dissolved in dichloromethane (12.5, 15, 17.5 ml). Three-level, two-factor full-factorial design optimized the above process. Next, the developed microsponges were thoroughly evaluated for entrapment efficiency, drug release and antimicrobial study. Lastly, film was developed which was made up of blend of bioadhesive polymers, namely pectin and xanthan gum. Subsequently, film was rolled into a torpedo-shaped capsule shell.
Results
The spherical-shaped porous microsponges exhibited a notable entrapment efficiency (94.17%), size 14.68 μm and rapid drug release of 91.1 ± 1.54% within 10 h. Further, bioadhesive film revealed thickness 92 μm, folding endurance 440, moisture content 5.66 ± 0.51% w/w, and good tensile strength to withstand vaginal pressure and remarkable zone of inhibition against Candida albicans (19.1 mm).
Conclusion
Fluconazole microsponges loaded bioadhesive film, rolled into a torpedo-shaped capsule, exhibited an excellent aesthetic appearance. Moreover, strong bioadhesivity ensured a high efficacy in combating disease.






Similar content being viewed by others
References
Rathod SD, Klausner JD, Krupp K, Reingold AL, Madhivanan P. Epidemiologic features of vulvovaginal candidiasis among reproductive-age women in India, infect. Dis Obstet Gynecol. 2012. https://doi.org/10.1155/2012/859071.
Johal HS, Garg T, Rath G, Goyal AK. Advanced topical drug delivery system for the management of vaginal candidiasis. Drug Deliv. 2016. https://doi.org/10.3109/10717544.2014.928760.
Saporiti AM, Gómez D, Levalle S, et al. Candidiasis vaginal: etiología y perfil de sensibilidad a agentes antifúngicos de uso clínico [Vaginal candidiasis: etiology and sensitivity profile to antifungal agents in clinical use]. Rev Argent Microbiol. 2001;33:217–22.
Pérez-González N, De Febrer NB, Calpena-Campmany AC, Nardi-Ricart A, Rodríguez-Lagunas MJ, Morales-Molina JA, Soriano-Ruiz JL, Fernández-Campos F, Clares-Naveros B. New formulations loading caspofungin for topical therapy of vulvovaginal candidiasis. Gels. 2021. https://doi.org/10.3390/gels7040259.
Rodero CF, Fioramonti Calixto GM, Cristina Dos Santos K, Sato MR, Aparecido Dos Santos Ramos M, Miró MS, Rodríguez E, Vigezzi C, Bauab TM, Sotomayor CE, Chorilli M. Curcumin-Loaded Liquid Crystalline systems for Controlled Drug Release and Improved Treatment of Vulvovaginal Candidiasis. Mol Pharm. 2018. https://doi.org/10.1021/acs.molpharmaceut.8b00507.
Hashemi SE, Shokohi T, Abastabar M, Aslani N, Ghadamzadeh M, Haghani I. Species distribution and susceptibility profiles of Candida species isolated from vulvovaginal candidiasis, emergence of C. Lusitaniae. Curr Med Mycol. 2019. https://doi.org/10.18502/CMM.5.4.2062.
Mendling W, El Shazly MA, Zhang L. Clotrimazole for vulvovaginal candidosis: more than 45 years of clinical experience. Pharmaceuticals. 2020. https://doi.org/10.3390/ph13100274.
Farr A, Effendy I, Frey Tirri B, Hof H, Mayser P, Petricevic L, Ruhnke M, Schaller M, Schaefer APA, Sustr V, Willinger B, Mendling W. Guideline: Vulvovaginal candidosis. Mycoses. 2021. https://doi.org/10.1111/MYC.13248.
Young GL, Jewell D. Topical treatment for vaginal candidiasis in pregnancy. Cochrane Database Syst Rev. 2000. https://doi.org/10.1002/14651858.CD000225.
Fan S, Liu X, Wu C, Xu L, Li J. Vaginal nystatin versus oral fluconazole for the treatment for recurrent vulvovaginal candidiasis. Mycopathologia. https://doi.org/10.1007/s11046-014-9827-4.
Sobel JD, Ferris D, Schwebke J, Nyirjesy P, Wiesenfeld HC, Peipert J, Soper D, Ohmit SE, Hillier SL. Suppressive antibacterial therapy with 0.75% metronidazole vaginal gel to prevent recurrent bacterial vaginosis. Am J Obstet Gynecol. 2006. https://doi.org/10.1016/J.AJOG.2005.11.041.
Francois M, Snoeckx E, Putteman P, Wouters F, De Proost E, Delaet U, Peeters J, Brewster ME. A mucoadhesive, cyclodextrin-based vaginal cream formulation of itraconazole. AAPS PharmSci. 2003. https://doi.org/10.1208/PS050105.
Phillips NA, Bachmann G, Haefner H, Martens M, Stockdale C. Topical treatment of recurrent Vulvovaginal Candidiasis: An Expert Consensus women’s Heal. Reports. 2022. https://doi.org/10.1089/WHR.2021.0065.
Govindarajan A, Bistas KG, Ingold CJ, Aboeed A. Fluconazole. 7th ed. Kucers The Use of Antibiotics. 2022; pp. 2756–2785. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024. Bookshelf ID: NBK537158.
Ghannoum MA, Rice LB. Antifungal agents: mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin Microbiol Rev. 1999. https://doi.org/10.1128/CMR.12.4.501.
Mishra R, Joshi P, Mehta T. Formulation, development and characterization of mucoadhesive film for treatment of vaginal candidiasis. Int J Pharm Investig. 2016. https://doi.org/10.4103/2230-973X.176487.
Singhvi G, Manchanda P, Hans N, Dubey SK, Gupta G. Microsponge: an emerging drug delivery strategy. Drug Dev Res. 2019. https://doi.org/10.1002/ddr.21492.
Tiwari A, Tiwari V, Palaria B, et al. Microsponges: a breakthrough tool in pharmaceutical research. Futur J Pharm Sci. 2022;8. https://doi.org/10.1186/s43094-022-00421-9.
Amrutiya N, Bajaj A, Madan M. Development of microsponges for topical delivery of mupirocin. AAPS PharmSciTech. 2009. https://doi.org/10.1208/S12249-009-9220-7.
Bothiraja C, Gholap AD, Shaikh KS, Pawar AP. Investigation of ethyl cellulose microsponge gel for topical delivery of eberconazole nitrate for fungal therapy. Ther Deliv. 2014. https://doi.org/10.4155/TDE.14.43.
Junqueira MV, Bruschi ML. A review about the drug delivery from Microsponges. AAPS PharmSciTech. 2018. https://doi.org/10.1208/S12249-018-0976-5.
Bhuptani RS, Patravale VB. Starch microsponges for enhanced retention and efficacy of topical sunscreen. Mater Sci Eng C. 2019. https://doi.org/10.1016/J.MSEC.2019.109882.
Wasilewska K, Winnicka K. Ethylcellulose-A Pharmaceutical Excipient with multidirectional application in Drug Dosage Forms Development. Mater. 2019. https://doi.org/10.3390/MA12203386.
Moin A, Deb T, Osmani RM, Bhosale R, Hani U. Fabrication, characterization, and evaluation of microsponge delivery system for facilitated fungal therapy. J Basic Clin Pharm. 2016. https://doi.org/10.4103/0976-0105.177705.
Pandit AP, Patel SA, Bhanushali VP, Kulkarni VS, Kakad VD. Nebivolol-loaded Microsponge Gel for Healing of Diabetic Wound. AAPS PharmSciTech. 2017. https://doi.org/10.1208/S12249-016-0574-3.
Bhatia M, Saini M. Formulation and evaluation of curcumin microsponges for oral and topical drug delivery. Prog Biomater. 2018. https://doi.org/10.1007/S40204-018-0099-9.
Obiedallah MM, Abdel-Mageed AM, Elfaham TH. Ocular administration of acetazolamide microsponges in situ gel formulations. Saudi Pharm J. 2018. https://doi.org/10.1016/J.JSPS.2018.01.005.
Khattab A, Nattouf A. Optimization of entrapment efficiency and release of clindamycin in microsponge based gel. Sci Rep. 2021. https://doi.org/10.1038/s41598-021-02826-7.
Sareen R, Nath K, Jain N, Dhar KL. Curcumin loaded microsponges for colon targeting in inflammatory bowel disease: fabrication, optimization, and in vitro and pharmacodynamic evaluation. Biomed Res Int. 2014. https://doi.org/10.1155/2014/340701.
Shahzad Y, Saeed S, Ghori MU, Mahmood T, Yousaf AM, Jamshaid M, Sheikh R, Rizvi SAA. Influence of Polymer ratio and surfactants on controlled drug release from cellulosic microsponges. Int J Biol Macromol. 2018. https://doi.org/10.1016/J.IJBIOMAC.2017.11.089.
Cirri M, Maestrelli F, Scuota S, Bazzucchi V, Mura P. Development and microbiological evaluation of chitosan and chitosan-alginate microspheres for vaginal administration of metronidazole. Int J Pharm. 2021. https://doi.org/10.1016/J.IJPHARM.2021.120375.
Osmałek T, Froelich A, Jadach B, Tatarek A, Gadzinski P, Falana A, Gralinska K, Ekert M, Puri V, Wrotynska-Barczynska J, Michniak-Kohn B. Recent advances in polymer-based Vaginal Drug Delivery systems. Pharmaceutics. 2021. https://doi.org/10.3390/PHARMACEUTICS13060884.
Klemetsrud T, Jonassen H, Hiorth M, Kjoniksen AL, Smistad G. Studies on pectin-coated liposomes and their interaction with mucin. Colloids Surf B Biointerfaces. 2013. https://doi.org/10.1016/J.COLSURFB.2012.10.012.
Karagianni A, Peltonen L. Production of Itraconazole Nanocrystal-based polymeric Film formulations for Immediate Drug Release. Pharmaceutics. 2020. https://doi.org/10.3390/PHARMACEUTICS12100960.
Kumar L, Reddy MS, Shirodkar RK, Pai GK, Krishna VT, Verma R. Preparation and characterisation of fluconazole vaginal films for the treatment of vaginal candidiasis. Indian J Pharm Sci. 2013;75:585–90.
Baloglu E, Ozyazici M, Hizarcioglu SY, Karavana HA. An in vitro investigation for vaginal bioadhesive formulations: bioadhesive properties and swelling states of polymer mixtures. Farmaco. 2003. https://doi.org/10.1016/S0014-827X(03)00044-2.
Alam MA, Ahmad FJ, Khan ZI, Khar RK, Ali M. Development and evaluation of acid-buffering bioadhesive vaginal tablet for mixed vaginal infections. AAPS PharmSciTech. 2007. https://doi.org/10.1208/PT0804109.
Akil A, ParniakMA, Dezzutti CS, Moncla BJ, Cost MR, Li M, Rohan LC. Development and characterization of a vaginal Film Containing Dapivirine, a non- nucleoside reverse transcriptase inhibitor (NNRTI), for prevention of HIV-1 sexual transmission. Drug Deliv Transl Res. 2011. https://doi.org/10.1007/S13346-011-0022-6.
Patel SK, Shah DR, Tiwari S. Bioadhesive films containing fluconazole for mucocutaneous candidiasis. Indian J Pharm Sci. 2015. https://doi.org/10.4103/0250-474X.151601.
Liu X, Ma Z, Zhang J, Yang L. Antifungal compounds against Candida Infections from Traditional Chinese Medicine. Biomed Res Int. 2017. https://doi.org/10.1155/2017/4614183.
Ammar HO, Ghorab MM, Mahmoud AA, Shahin HI. Design and in Vitro/In vivo evaluation of Ultra-thin Mucoadhesive Buccal Film Containing Fluticasone Propionate. AAPS PharmSciTech. 2017. https://doi.org/10.1208/s12249-016-0496-0.
Li J, Regev G, Patel SK, Patton D, Sweeney Y, Graebing P, Grab S, Wang L, Sant V, Rohan LC. Rational Design of a Multipurpose Bioadhesive Vaginal Film for Co-Delivery of Dapivirine and Levonorgestrel. Pharmaceutics. 2020. https://doi.org/10.3390/PHARMACEUTICS12010001.
Germini G, Peltonen L. 3D Printing of Drug Nanocrystals for Film formulations. Molecules. 2021. https://doi.org/10.3390/molecules26133941.
Martín-Illana A, Cazorla-Luna R, Notario-Pérez F, Rubio J, Ruiz-Caro R, Tamayo A, Veiga MD. Eudragit® L100/chitosan composite thin bilayer films for intravaginal pH-responsive release of Tenofovir. Int J Pharm. 2022. https://doi.org/10.1016/J.IJPHARM.2022.121554.
Krull SM, Patel HV, Li M, Bilgili E, Davé RN. Critical material attributes (CMAs) of strip films loaded with poorly water-soluble drug nanoparticles: I. Impact of plasticizer on film properties and dissolution. Eur J Pharm Sci. 2016. https://doi.org/10.1016/J.EJPS.2016.07.005.
Cetindag E, Pentangelo J, Arrieta Cespedes T, Davé RN. Effect of solvents and cellulosic polymers on quality attributes of films loaded with a poorly water-soluble drug. Carbohydr Polym. 2020. https://doi.org/10.1016/J.CARBPOL.2020.117012.
Pandit A, Kedar A, Koyate K. Hollow pessary loaded with lawsone via self-microemulsifying drug delivery system for vaginal candidiasis. J Drug Delivery Sci Tech. 2020. https://doi.org/10.1016/J.JDDST.2020.101955.
Park T, Lee S, Amatya R, Maharjan P, Kin H, Park W, Ahn M, Kim S, Moon C, Cheong H, Min K, Shin M. Development and characterization of a superabsorbing hydrogel film containing Ulmus davidiana var. Japonica root bark and pullulan for skin wound healing. Saudi Pharm J. 2020. https://doi.org/10.1016/j.jsps.2020.05.007.
Acarturk F. Mucoadhesive Vaginal Drug Delivery systems. Recent Pat Drug Deliv Formul. 2009. https://doi.org/10.2174/187221109789105658.
Acknowledgements
Authors thanked Associated Capsules Group Pvt. Ltd., Mumbai, India for providing torpedo shaped capsules generously.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest regarding the publication of this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mandlik, P.L., Lad, P.R. & Pandit, A.P. Fluconazole Microsponges Loaded Bioadhesive Vaginal Film to Treat Vulvovaginal Candidiasiss. J Pharm Innov 19, 12 (2024). https://doi.org/10.1007/s12247-024-09822-0
Accepted:
Published:
DOI: https://doi.org/10.1007/s12247-024-09822-0