Abstract
Purpose
Monoclonal antibodies (mAbs) are important active ingredients of molecularly targeted drugs, which are only effective for specific patient groups. Early assessment of their effectiveness is important for more efficient use of time and resources. Companion diagnostics (CDx) are medical devices or tests to identify groups of promising patients based on specific biomarkers. This work offers a systems evaluation model and a comprehensive assessment from multiple stakeholder perspectives.
Methods
This work introduces a new systems model for assessing available treatment options. Process system diagrams, consisting of independently defined unit structures, are applied to represent the expected decision points and outcomes. A sensitivity analysis is conducted to identify the critical requirements for achieving cost-effectiveness. The model was applied to a case of terminal colorectal cancer treatment to compare mAb drugs to standard therapy.
Results
The results showed that from the payers’ perspective, the cost and response rates of the mAb drug were critical parameters to improve for achieving the target cost-effectiveness. The results give quantitative guidance for the required improvement.
Conclusion
This work represents an important step towards a fair and systematic assessment of treatment alternatives and serves as a guideline for future CDx and therapy technology development efforts.
Similar content being viewed by others
Teaser
This work presents a systems evaluation model for assessing the cost-effectiveness of emerging CDx and molecular targeted therapies. The model gives quantitative guidance for the required improvement in these technologies.
Introduction
Monoclonal antibodies (mAbs) represent an important category of biopharmaceuticals widely used in molecularly targeted therapy [1]. Molecularly targeted therapies are designed to be more effective for patients with specific features [2]. As such, they are expected to be easier to personalize and to better target diseases which have been difficult to cure with conventional therapies [3]. On the other hand, their high costs have been a subject of controversy, which introduces the need for a targeted approach for effective use [4, 5]. Therefore, to enhance the cost-effectiveness of mAb drugs, it is important to identify in advance patient groups who are more likely to show a positive response or lower side effects to the therapy.
Companion diagnostics (CDx) are medical devices or tests that provide essential information to identify groups of promising patients based on the specific biomarkers [6, 7]. Here, biomarkers are factors that can be objectively assessed as an indicator of pharmacological reactions to a therapeutic intervention [8], e.g., genetic mutations or specific proteins on cancer cells. Various types of devices for CDx have also been developed and approved for different molecularly targeted therapies [9, 10]. In Japan, for example, the application of cetuximab (ERBITUX®) to colorectal cancer has three approved CDx devices to investigate the presence of the KRAS/NRAS genetic mutation. Patients without the mutation are expected to show more positive responses to cetuximab than those with the mutation [11]. Harty et al. [11] and Shiroiwa et al. [12] assessed the cost-effectiveness of CDx for colorectal cancer treatment and showed that the stratified use of cetuximab based on testing (K)RAS mutation could provide better cost-effectiveness than random use. CDx is expected to maximize patient benefit while improving cost-effectiveness. However, in order to guarantee such advantages of CDx, assessing the total risk of treatments including CDx is necessary.
There are several risk factors in a treatment process including CDx. The first is the accuracy of the CDx. This paper differentiates between two accuracies. The accuracy of detecting the target biomarker (henceforth termed “detection accuracy”) and the effectiveness of the biomarker to predict response to the treatment (henceforth termed “response rate”). A low detection accuracy or response rate can lead to incorrect diagnosis and treatment. The waste of time and resources could be critical, especially for patients with short life expectancy. Moreover, inappropriate implementation of any therapy may adversely affect the health of patients due to its side effects. The second risk factor is the physical burden of CDx. Some CDx require samples to be taken through a highly invasive process, such as a tumor tissue biopsy. For patients with serious medical conditions, this can be a lethal burden or a limitation to further diagnosis and treatment. The third risk factor is the costs of the entire treatment including CDx. Depending on the features of each disease, the type and number of required CDx processes vary to identify the targeted biomarkers. Repetitions of CDx applications only exacerbate the associated risks including psychological, physical, and financial burdens to the patient. For a fair assessment of the application of CDx, the probability of treatment outcomes should be evaluated considering these risk factors.
The cost–benefit analysis of CDx applications has been discussed in several research works. For example, Schluckebier et al. [13] assessed the cost-effectiveness of several CDx tests including next generation sequencing (NGS) in case of lung cancer treatment. NGS is a technology scanning a person’s entire genome instead of testing specific genetic mutations like conventional CDx. Schluckebier et al. [13] showed that in the case of Brazil, the CDx with NGS was not cost-effective compared to conventional CDx technologies, despite its higher precision. This work highlighted the need for evaluating the probabilities of different risk factors independently. Seo and Cairns [14] summarized the findings from 22 other recent studies on the analysis of the cost-effectiveness of CDx for cancer treatments. The review emphasized the challenges hindering the fair estimation of overall benefits and risks. One of the major challenges reported was the lack of consensus between different studies and evaluation methods. For example, many existing works focused only on specific patient groups, whose biomarker status has already been identified. Leading to underestimating the costs of CDx compared to the obtained benefits. Despite the existence of several stakeholders in the system, most financial estimates were conducted from a third-party payer perspective rather than a societal perspective. Studies often miss important scenarios for forming comprehensive overviews, for example, comparing stratified use to complete the use of novel CDx or therapies and missing scenarios of only using standard of care (SOC) without conducting CDx. However, the use of CDx is not a trivial decision point considering the number of available and emerging options and the varying degrees of patient invasiveness and discomfort involved. Other challenges in existing works include difficulties in estimating uncertainties, clinical information such as the prevalence of biomarkers and the clinical resources, the impacts of timing of test use, and patient preference. The narrow perspectives adopted can lead to dismissal of risks or potential benefits. Therefore, a system evaluation model is still required to overcome these challenges.
An additional challenge is how to reflect potential future improvements. Various new technologies are continuously developed to mitigate risks, e.g., new CDx technologies aiming at higher detection accuracy, less physical burden by using body fluids instead of tumor tissues, or new therapies aiming at higher response rates [15]. However, previous reports of economic evaluation have mainly focused on currently available technologies, not future technologies. The cost-effectiveness can be affected by improvements in various parameters, e.g., accuracy of CDx or effectiveness of therapies [13, 16]. Conducting prospective and quantitative analyses can help guide improvement efforts by identifying the most influential factors in the decision-making process from a global systems perspective.
This work aims to present a systems evaluation model to assess the overall benefits and risks while providing guidance for promising future directions. The model consists of different unit operations to describe actions like applying CDx, mAb therapy, or alternative standard therapy at different splitters (e.g., decision points). The work aims to overcome the previously mentioned challenges through the application of a process systems framework with independent modules for additional flexibility in the assessment. A case study is presented for the cetuximab application to colorectal cancer. The outcomes of 20 individual paths and four comprehensive scenarios are evaluated depending on the initial decision factors. The best performing scenarios are identified from different perspectives for individual patients and for the payers. Furthermore, promising directions for the required improvements to achieve the target cost-effectiveness are presented. This work thus represents an important step for realizing a fair and comprehensive assessment of the total benefits and risks in the system.
Methodology
Overview of the Diagnostics and Therapy Process
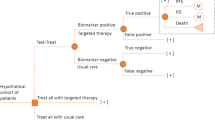
Figure 1 shows an overview of a typical diagnostics and therapy process for an individual patient with two therapy options. There are several decision points following disease diagnosis (red splitters in Fig. 1). If a targeted therapy option (Therapy A in Fig. 1) is associated with a corresponding CDx, the patient will first decide whether to take the CDx (the first red splitter in Fig. 1). In the case of a positive CDx result, the patient will expect Therapy A to be effective and is assumed to automatically opt for it (the automatic decision point is marked by a green splitter in Fig. 1). In the case of a negative result, a second decision point comes into play (the second red splitter in Fig. 1), where the patient can actively choose an alternative therapy (Therapy B in Fig. 1) or decline all therapy options. If the patient declined the CDx at the first decision point, then the following decision point involves the choice between Therapies A, B, or no treatment. The dotted region in Fig. 1 is the main focus of this work.
Description of the Diagnostics and Therapy Process as a Process System Diagram
The dotted region in Fig. 1 is expanded, and a higher resolution pathway is presented in Fig. 2a as a process system diagram. The diagram describes 20 possible patient paths and the split rules leading to each outcome. Each split is defined based on a patient’s decision and/or biological features. Figure 2b presents detailed descriptions of the unit structures used to build the process system diagram in Fig. 2a. Unit structures include splitters, unit operations, and agents. Decision-making splitters are indicated in red and represent active decision points (\({\mathrm{D}}_{i}\)). The outcome path for a patient is assumed to change based on subjective decisions made at those split points. In contrast, outcome paths will be automatically decided based on biological or statistical features in the other splitters. Biomarker splitters in grey (\({\mathrm{B}}_{i}\)) indicate the presence or absence of a particular biomarker, where \(x\) [–] represents the ratio of patients with positive biomarkers in a general patient population. Companion diagnostic splitters in green (\({\mathrm{X}}_{i}\)) indicate the ability of a CDx to detect the biomarker. \(\alpha\) [–] is the probability of type 2 error (false positive), and \(\beta\) [–] is the probability of type 1 error (false negative) in the obtained CDx results. Finally, the therapy splitters in purple (\({\mathrm{R}}_{i}\)) indicate the probability of the therapy working effectively for a patient. \(\gamma\) [–] is the response rate, indicating the probability of a positive response on the application of a specific therapy for the patient. “Unit operations” in Fig. 2b indicates the different potential unit operations: perform companion diagnostics, apply molecular targeted therapy, e.g., mAb therapies, or apply standard therapy. If several molecular targeted or standard therapies exist, then a new unit operation could be added for each existing therapy option. A path with no unit operations indicates a decision to not undergo any treatment. \(\varepsilon\) [–] is a factor representing the change in the life expectancy at diagnosis following the performance of each unit operation relative to the no-action path. The subscripts “t” and “s” represent the targeted and standard therapies, respectively. Values for \(\gamma\) and \(\varepsilon\) are determined for each patient based on the patient’s biological features and general statistical effectiveness of each therapy for different patient groups. “Agents” in Fig. 2b indicates the agent impacted by the process.
Figure 2a shows the complete process system diagram for the case, where one mAb therapy with one CDx is compared to standard therapy or no treatment options. Different outcomes are expected based on the biological features of each patient. For example, the no-action path will yield different life expectancy for patients with and without target biomarkers. Important splitters in the figure include (1) \({\mathrm{D}}_{1}\), a patient’s decision to take CDx; (2) \({\mathrm{B}}_{1}\), the patient possessing a positive biomarker; (3) \({\mathrm{X}}_{1}\), the result of the CDx; (4) \({\mathrm{R}}_{1}\), a patient’s response to the targeted therapy (similarly \({\mathrm{R}}_{2}\), a patient’s response to the standard therapy); (5) \({\mathrm{D}}_{2}\), the patient’s decision whether to undergo the targeted therapy without undergoing CDx; (6) \({\mathrm{D}}_{3}\), decisions to terminate the therapy or return to a previous decision point (e.g., \({\mathrm{D}}_{1}\) to take the same or a different type of CDx); (7) \({\mathrm{D}}_{4}\), decisions to take the standard therapy. In the figure, the superscripts “1” and “2” indicate biomarker-positive and negative patients, respectively. The subscripts “e” and “ne” indicate patients with a positive or a negative response to a particular therapy, respectively. In total, 20 potential outcomes for changes in the life expectancy are identified for this system, each outcome is defined in the figure with a unique path ID.
Individual Paths and Scenarios
Individual patient paths are those determined by a patient’s decisions and features (e.g., biomarker-positive or negative). The different paths, 1 to 20 in Fig. 2a, correspond to the different potential outcomes for each patient. The determination of the likelihood of a patient achieving a specific outcome once their biological features are identified would be useful in the decision-making process for each patient. On the other hand, a scenario is a group of those paths as defined only by decision points (the red splitters). The evaluation of different scenarios would be useful for both patients who do not have enough information about their biological features and payers who want to evaluate the overall system. Both individual paths and group scenarios are the targets of evaluation in this work.
Evaluation Indicators
For Individual Paths
The life expectancy at diagnosis for each individual path, \({LY}_{\mathrm{after}}\) (year), the total cost, \({C}_{\mathrm{total},\mathrm{indiv}}\) (Japanese yen (hereafter referred to as JPY)), and the total cost per year of life expectancy for each path, \(CE\) (JPY year−1), were calculated, as shown in Eqs. (1)–(3), respectively.
where \({LY}_{\mathrm{before}}\) (year) is the life expectancy at diagnosis in the no-action path for each patient. This value may vary depending on the existence of specific biomarkers (difference between paths 19 and 20 in Fig. 2a). The overall \(\varepsilon\) will be greater than 1 for paths with effective combinations of therapies and diagnostics and smaller than 1 for paths with negative overall effects due to the side effects. \(\varepsilon\) is 1 for the ineffective paths or for the paths where no action is taken.
The total cost of an individual path includes the costs of any therapies and applied CDx (\({C}_{\mathrm{CDx}}\) (JPY)). \({t}_{\mathrm{therapy}}\) (month) is the minimum of the expected remaining lifetime for each patient or the medically recommended duration of each therapy option. \({C}_{\mathrm{therapy}}\) (JPY month−1) is the total monthly cost of each applied therapy. Therefore, \({C}_{\mathrm{therapy}}\) can be either \({C}_{\mathrm{target}}\) (JPY month−1) (the cost for targeted therapy per month) or \({C}_{\mathrm{standard}}\) (JPY month−1) (the cost for standard therapy per month) or their sum depending on the recommended medical action path for each disease. A path with a higher \(CE\) value, thus, reflects a less cost-effective option.
Previous studies have commonly used the incremental cost-effectiveness ratio (ICER) as the cost-effectiveness indicator [16]. ICER is generally used to judge the feasibility of an investment by comparing the difference between new and standard applications. For pharmaceutical applications, it is calculated via the evaluation of the additional costs (e.g., \(\Delta {C}_{\mathrm{total},\mathrm{indiv}}\)) incurred to achieve a positive outcome (e.g., \(\Delta {LY}_{\mathrm{after}}\)) using new technologies (or drugs) relative to the standard technologies which are commonly used. The standard technologies are regarded as the baseline. However, in this work, there are multiple baselines involved even with the application of the standard therapy (e.g., with or without a biomarker or genetic mutation, or showing response to the therapy or not), which makes IECR difficult to apply and compare. Therefore, the individual \({LY}_{\mathrm{after}}\) and \({C}_{\mathrm{total},\mathrm{indiv}}\) were used to calculate the effectiveness of each path instead of defining a specific baseline.
Scenario Definitions
Since scenarios are the groups of several paths which are the potential outcomes of the same decisions, each scenario was evaluated through the expectation of the evaluation indicators of its underlying paths as follows in Eqs. (4) and (5):
where \({i}_{{j}_{s}}\) and \({p}_{{j}_{s}}\) [–] are the evaluation indicator value and the probability of the potential patient path \(j\) in scenario \(s\), respectively. \({j}_{s,\mathrm{min}}\) and \({j}_{s,\mathrm{max}}\) are the minimum and the maximum path ID representing the range of potential patient paths belonging to scenario \(s\), respectively. \(E\left({I}_{s}\right)\) is the expectation value of the indicator \(I\), for scenario \(s\).
The probability of each the potential patient path \(j\) was calculated based on the values set for each splitter. For example, the ratio of patients with positive biomarkers in a general population was used to set x [–] for \({\mathrm{B}}_{i}\). Depending on the set values and assumptions taken, there could be some paths which no patient could reach. Such paths were called zero-paths while the others were called non-zero paths.
Model Assumptions
First, the gradual change in the patient’s health status during the therapy over time was not considered. The effect of therapy was assumed to be achieved at once upon application. In this work, only the cost of therapy and diagnostics was included, but the cost of care during therapy was not considered. A longer treatment process might incur higher personal care costs in that duration. Second, the effectiveness of therapy was only varied based on differences in patient groups (i.e., with or without biomarkers and showing response to therapy or not). Individual differences between patients on the same path were neglected. The effect of therapy was assumed to be constant and identical for everyone going through the same path.
Model Applications
In addition to the patient, a medical doctor, CDx researcher, clinical researcher, and government can be involved as the stakeholders in the system. Model parameters can be updated based on new inputs from different stakeholders. For example, new or improved therapies developed by clinical researchers can affect the determination of the response rate and the estimated change in life expectancy (\(\gamma\) and \(\varepsilon\)), respectively. The model can be expanded to compare the impact of all new therapies on the outcomes for different patient groups. Furthermore, the values of \(\gamma\) could also be affected through the identification of new biomarkers, more closely related to the success of the targeted therapy. Developments by CDx researchers to improve biomarker detection accuracy will affect the values of \(\alpha\) and \(\beta\). The developed model thus provides a standardized method for the comparison of novel developments to existing alternatives. This flexibility provides a systematic and comprehensive framework for quantifying the effects of any developments in the system on all expected outcomes for each patient. Various stakeholders can use the model from different perspectives. For example, the model can be applied to assess all possible outcomes for a single patient, or for entire groups of patients (e.g., by the government), which could be highly beneficial for policy setting or for guiding future research directions.
Sensitivity Analysis
Sensitivity analyses were conducted with the following two objectives: (1) to identify the critical parameters in the system and (2) to quantify the required improvement in the identified critical parameters by assessing the necessary directions to achieve the desired cost-effectiveness. First, a local sensitivity analysis was conducted, where each parameter was varied in a range of 10% of the base case in the direction of a more favorable performance (e.g., reduction in mAb cost, increase in response rate). Then, the parameters with the largest impact on changes in cost-effectiveness were identified. Second, those identified parameters were varied simultaneously in the range of ± 100%. In cases of division by 0, the minimum value was taken as − 99% instead. The upper limit was replaced by the maximum parameter value in cases where + 100% was not feasible. The boundaries were set relatively far from the nominal values to fully investigate any potential changes in cost-effectiveness with future development of the identified influential parameters. All calculations were conducted using inhouse developed Python codes.
Case Study
Case Study Settings
A case study was performed for the application of cetuximab to colorectal cancer at stage 4 (unresectable, progressive, or recurrent.). Here, the targeted therapy was the application of cetuximab in combination with standard chemotherapy involving the application of FOLFIRI (irinotecan, fluorouracil, and leucovorin). Both subscripts “target” of \({C}_{\mathrm{target}}\) and “t” of \({\gamma }_{\mathrm{t}}\) and \({\varepsilon }_{\mathrm{t}}\) were changed to “mab” for immediate interpretations. The standard therapy route in this case involved FOLFIRI alone. CDx in this case was for testing the presence of KRAS mutations in the DNA of cells in the tumor tissue. The use of MEBGEN RASKET™-B kit was assumed for testing. Relevant parameter values including the life expectancy for different patient groups were set as shown in Table 1 based on the previous works. In the case of the detection rate, since the detection of the genetic mutation was relatively precise, both the false ratio \(\alpha\) and \(\beta\) were assumed to be zero. Lastly, the application of the CDx was assumed to have no negative effects on life expectancy.
Scenario Setting
Four main scenarios define the active decision-making points and result in the 20 potential outcome paths. The details of each scenario are shown in Table 2: (A) take CDx and therapy accordingly based on the result of CDx; (B) take mAb therapy without CDx; (C) forgo CDx and opt for standard therapy; and (D) forgo any therapies. The potential path will be uniquely specified based on a patient’s biological features when one of the four scenarios is chosen. In this case study, two assumptions were made as follows: (1) after taking CDx, the CDx results determined the subsequent therapy, and there was no active decision to decline all therapies (\({\mathrm{D}}_{4}\) in Fig. 2b, thus the probability of paths 5 and 10 was set to zero, making them zero paths); (2) the upper limit of \({t}_{\mathrm{therapy}}\) was 6 months and if the \({LY}_{\mathrm{after}}\) is shorter than that, the therapy was assumed to be terminated at the end of \({LY}_{\mathrm{after}}\), so there was no active decision regarding therapy termination (\({\mathrm{D}}_{3}\) in Fig. 2b) in this case study.
Results and Discussion
Results for Individual Paths
Table 3 shows the total cost per year of life expectancy \(CE\) of the individual non-zero paths except for paths 19 and 20, where no treatment was performed. In this case study, zero paths were paths 3, 4, 6, and 7 due to the assumption of the perfect detection accuracy of CDx in addition to paths 5 and 10 due to the assumption of Scenario A. For all cases, Scenario C was the most cost-effective. For a meaningful comparison of the results for individual patients, the effect of different decisions should only be compared within the same patient group with similar biological features. For example, Scenarios A and B are equivalent in terms of cost-effectiveness for patients with no mutation and showing a positive response to the cetuximab therapy (paths 1 and 11). This indicates that, in this case, the cost of CDx is negligible compared to the cost of the therapy itself. On the other hand, when a patient has the mutation, Scenario A was an order of magnitude more cost-effective than Scenario B because Scenario A could avoid the ineffective use of cetuximab by following the result of CDx. This shows that the detection accuracy of the mutation is a critical factor for the payers assessing the overall system.
Results for Scenarios
Figure 3 shows the results of the scenario analysis. The highest average life expectancy was achieved in Scenario A followed closely by Scenario B (Fig. 3a). A significant improvement in life expectancy can be expected with Scenarios A, B, and C compared to the no-action path in Scenario D (about 4.7, 4.6, and 4.2 times, respectively). The difference between scenarios in which cetuximab could be used (Scenarios A and B) and the scenario in which only standard therapy was used (Scenario C) indicates a stronger impact of cetuximab compared to the standard therapy.
A similar trend was observed in Fig. 3b for the total cost \({E(C}_{\mathrm{total},\mathrm{indiv}})\) as that shown in Fig. 3c for the total cost per life expectancy \(E(CE)\). Between Scenarios A and B, Scenario A (taking CDx) showed higher cost-effectiveness than Scenario B (not taking CDx). This is due to the ability to avoid the unnecessary use of cetuximab for patients who would show no response. Scenario C (only standard therapy) resulted in the best cost-effectiveness, among the scenarios in which therapy was performed. Although the evaluation indicator values were different, the trends in the scenario analysis were consistent with previous studies; the CDx made the corresponding therapy more cost-effective but not as much as the alternative therapy [27].
Based on the scenario analysis results, Scenario A gave the highest life expectancy, while Scenario C gave the best cost-effectiveness. It indicated that the scenario which is the best for a patient is not necessarily the best for payers (e.g., government). Other factors could also affect the decisions in this case, which were not addressed in this work. For example, the quality of life for the patient with each treatment could vary depending on the expected side effects impacting patient preferences.
Improvement Opportunities for Individual Paths
Table 4 shows an example of potential improvements to make the paths in Scenario A as cost-effective for patients without the genetic mutation as those in Scenario C. In order to improve the cost-effectiveness of individual paths, the change in the life expectancy \({\varepsilon }_{\mathrm{e},\mathrm{ mab}}\) should be enhanced and/or the total cost reduced. The influential factors on the total cost are the therapy duration and/or the costs of CDx and cetuximab. Reductions in therapy duration, for example, can be achieved through earlier decisions to terminate the therapy in cases with no positive response. Given the big difference in the price between the targeted and the standard therapies (\({C}_{\mathrm{mab}}\) and \({C}_{\mathrm{standard}}\) in Table 1) and the relatively small difference in life expectancy achieved between them, the targeted therapy cannot easily become cost-effective as shown in Table 4. The most favorable case can be achieved, where a response is only possible through the targeted therapy (case II). The higher costs of this therapy still require a 66.5% reduction in cost to be comparable in terms of cost-effectiveness to that of the standard therapy, even with no response and no additional extension of life expectancy. Alternatively, a 2.99 factor improvement of \({\varepsilon }_{\mathrm{e},\mathrm{ mab}}\) would achieve the same results. In cases where a response can be achieved by the standard therapy (case I), the required factors of change become much higher. In cases with no expected response from the targeted therapy, the additional cost is not warranted and cannot be made comparable to the standard therapy. Therefore, paths in Scenario A are not cost-effective for individual patients. In this case, further development of different targeted therapies would be required to either achieve further benefits or avoid high costs.
Sensitivity Analysis for Scenarios
Table 5 shows the results of the local sensitivity analysis to changes in the parameter values in Scenario A. From the results, cetuximab response rate for patients without KRAS mutation \({\gamma }_{\mathrm{mab}}^{1}\) and cost \({C}_{\mathrm{mab}}\) were identified as critical variables having the largest impact on the cost-effectiveness \(CE\). A further sensitivity analysis was conducted to examine the required changes in those parameters to make Scenario A as cost-effective as Scenario C.
Figure 4 shows the results of varying the cetuximab response rate and the cost for Scenario A. The cetuximab response rate \({\gamma }_{\mathrm{mab}}^{1}\) was varied in the range of − 100% (no patient showed response) to 70% (all patients showed response) of the base case (X-axis) to reach the maximum value of 1. The cost \({C}_{\mathrm{mab}}\) was changed in the range of − 100% (free of charge) to 100% (double price) of the base case (Y-axis). For this analysis, both values were varied in the increments of 1%. The impact of changes in these parameters was tested on the life expectancy with Scenario A \(E\left({LY}_{\mathrm{after}}\right)\) shown in Fig. 4a, the total costs \(E\left({C}_{\mathrm{total},\mathrm{indiv}}\right)\) shown in Fig. 4b, and cost-effectiveness \(E\left(CE\right)\) shown in Fig. 4c.
Results of sensitivity analysis for Scenario A (cetuximab response rate \({\gamma }_{\mathrm{mab}}^{1}\) and cost \({C}_{\mathrm{mab}}\)) in terms of a life expectancy at diagnostics, b total cost, and c total cost per life expectancy. The red line and area below give the comparable expectation of the cost-effectiveness as Scenario C
Since \({LY}_{\mathrm{after}}\) was assumed not to be a function of any financial costs, it changed in conjunction with the cetuximab response rate only (Fig. 4a). On the other hand, the total costs changed in conjunction with both mAb response rate and mAb cost (Fig. 4b). At large enough reductions in the mAb cost, the change in total costs becomes almost independent of the change in response rate. Improvements in the response rate are more critical for treatments of higher costs. As expected, the cost-effectiveness of the scenario improved with higher values of the response rate and lower mAb costs. The area where Scenario A can be considered cost-effective as Scenario C was very small, as marked in Fig. 4c (red line and the area below). To achieve higher cost-effectiveness than Scenario C, the mAb cost should be reduced by 88% at least. However, it does not have to be as inexpensive as the standard therapy because of the mitigation of some risks (e.g., avoiding wasted time with ineffective therapy for patients with relatively small \(LY\)). This insight would help the CDx and clinical researchers including pharmaceutical companies and the government to set the goal of developing new options and revision of drug prices.
The development of more effective treatments can lead to improvements in the response rate and/or life expectancy. Therefore, the sensitivity of the results to further changes in the life expectancy with cetuximab \({\varepsilon }_{\mathrm{e},\mathrm{ mab}}\) was also investigated. A similar analysis was conducted, where \({\varepsilon }_{\mathrm{e},\mathrm{ mab}}\) and cetuximab cost \({C}_{\mathrm{mab}}\) were varied, as shown in Fig. 5. A similar trend to that in Fig. 4a was observed in Fig. 5a, where changes in the expectation value of life expectancy in Scenario A were not a function of changes in cost and only depended on changes in \({\varepsilon }_{\mathrm{e},\mathrm{ mab}}\). When the life expectancy exceeded that recommended treatment duration, it was assumed that the treatment was terminated successfully, which leads to a stabilization. This is seen in Fig. 5b, where the change in life expectancy has no further impact on the total costs after a certain threshold. Comparable cost-effectiveness of Scenarios A and C could not be achieved by varying the life expectancy in a realistic range. From an overall perspective (e.g., the payer’s), improving the response rate has a stronger influence than extending the potential life expectancy for a limited patient group.
Advantage of the Applied Method
The advantage of the applied method is its flexibility. Depending on the stakeholder and their interest, the perspective of the analysis can flexibly and objectively change. For individual patients, it is always better to recognize as many biological features in advance, as this could greatly affect the results. The applied method can provide the outcomes of the individual paths, and the patients can make more informed decisions if they can identify their biological features. However, even when patients do not know such biological features, the method can provide decision-support by providing the expectation value for each scenario. For policy-making and future research efforts, the expectation could work to provide future directions and to judge the overall benefit to society.
Conclusions and Outlook
In this work, a systems evaluation model was presented to assess the overall benefits and risks while providing guidance for promising future directions. The model consists of different unit structures. Through the application of a process systems diagram with independent modules, additional flexibility in the assessment was obtained. A systems analysis was provided, where each decision point was parameterized. Through the sensitivity analysis, a quantitative assessment of the influential factors to be improved could be obtained. As a result of the case study, promising directions for the required improvements to achieve the target cost-effectiveness were presented for the case of colorectal cancer.
Future work includes considering time factors in the model. It would enable the reflection of changes in decisions based on the intermediate results of the diagnosis and the therapy. In addition, expanding evaluation indicators could provide further insights, for example, by including indicators such as disability-adjusted life year (DALY) [28, 29] or quality-adjusted life year (QALY). It would allow considering the patients’ physical burden associated with treatment (e.g., pain, nausea) and the emotional distress (e.g., hair loss) caused by side effects. Then, it would lead to a more versatile and more practical method for considering patient benefits depending on features of therapy.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- \({C}_{\mathrm{CDx}}\) :
-
Cost of companion diagnostics (JPY time−1)
- \({C}_{k}\) :
-
Cost of therapy k (JPY month−1)
- \({C}_{\mathrm{total},\mathrm{ indiv}}\) :
-
Total cost for one patient (JPY)
- \(CE\) :
-
Cost-effectiveness (JPY year−1)
- \(E\left(I\right)\) :
-
Expectation of \(I\) (year), (JPY), or (JPY year−1)
- I :
-
Arbitral evaluation indicator (year), (JPY), or (JPY year−1)
- \({i}_{j}\) :
-
Evaluation indicator value of the potential patient path \(j\) (year), (JPY), or (JPY year−1)
- \(LY\) :
-
Life expectancy (year)
- \({p}_{j}\) :
-
Probability of going to the potential patient path \(j\) (–)
- \(t\) :
-
Time (month)
- \(x\) :
-
Ratio of patients having positive biomarker (expected to be effective) (–)
- \(\alpha\) :
-
Ratio of type 2 error (–)
- \(\beta\) :
-
Ratio of type 1 error (–)
- \(\gamma\) :
-
Response rate of therapy (–)
- \(\varepsilon\) :
-
Change in the life expectancy (–)
- 1:
-
Biomarker-positive
- 2:
-
Biomarker-negative
- after:
-
After going through the process
- before:
-
Before going through the process
- CDx:
-
Companion diagnostics
- e:
-
Effective
- f:
-
First month
- i :
-
Splitter i
- indiv:
-
Per individual patient
- j :
-
Path j
- LY:
-
Life expectancy
- mab:
-
Monoclonal antibody
- ne:
-
Not effective
- s :
-
Scenario
- s:
-
Standard therapy
- standard:
-
Standard therapy
- t:
-
Targeted therapy
- target:
-
Targeted therapy
- therapy:
-
Therapy
- total:
-
Total
- w:
-
Patients with KRAS mutation
- wo:
-
Patients without KRAS mutation
References
Charlton P, Spicer J. Targeted therapy in cancer. Medicine (United Kingdom). 2016;44(1):34–8. https://doi.org/10.1016/j.mpmed.2015.10.012.
Lee YT, Tan YJ, Oon CE. Molecular targeted therapy: treating cancer with specificity. Eur J Pharmacol. 2018;834(June):188–96. https://doi.org/10.1016/j.ejphar.2018.07.034.
Zahavi D, Weiner L. Monoclonal antibodies in cancer therapy. Antibodies. 2020;9(3):34. https://doi.org/10.3390/antib9030034.
Annett S. Pharmaceutical drug development: high drug prices and the hidden role of public funding. Biol Futur. 2021;72(2):129–38. https://doi.org/10.1007/s42977-020-00025-5.
Wu AC, Fuhlbrigge AL, Robayo MA, Shaker M. Cost-effectiveness of biologics for allergic diseases. J Allergy Clin Immunol: In Pract. 2021;9(3):1107-1117.e2. https://doi.org/10.1016/j.jaip.2020.10.009.
Papadopoulos N, Kinzler KW, Vogelstein B. The role of companion diagnostics in the development and use of mutation-targeted cancer therapies. Nat Biotechnol. 2006;24(8):985–95. https://doi.org/10.1038/nbt1234.
U.S. Food and Drug Administration. Companion diagnostics. 2018. https://www.fda.gov/medical-devices/in-vitro-diagnostics/companion-diagnostics.
Puntmann VO. How-to guide on biomarkers: biomarker definitions, validation and applications with examples from cardiovascular disease. Postgrad Med J. 2009;85(1008):538–45. https://doi.org/10.1136/pgmj.2008.073759.
Jørgensen JT. The current landscape of the FDA approved companion diagnostics. Transl Oncol. 2021;14(6). https://doi.org/10.1016/j.tranon.2021.101063.
V. Valla, S. Alzabin, A. Koukoura, A. Lewis, A. A. Nielsen, and E. Vassiliadis, “Companion diagnostics: state of the art and new regulations,” Biomarker Insights, vol. 16. SAGE Publications Ltd, 2021. doi: https://doi.org/10.1177/11772719211047763.
Harty G, Jarrett J, Jofre-Bonet M. Consequences of biomarker analysis on the cost-effectiveness of cetuximab in combination with FOLFIRI as a first-line treatment of metastatic colorectal cancer: personalised medicine at work. Appl Health Econ Health Policy. 2018;16(4):515–25. https://doi.org/10.1007/s40258-018-0395-5.
Shiroiwa T, Motoo Y, Tsutani K. Cost-effectiveness analysis of KRAS testing and cetuximab as last-line therapy for colorectal cancer. Mol Diagn Ther. 2010;6(14):375–84.
Schluckebier L, et al. Cost-effectiveness analysis comparing companion diagnostic tests for EGFR, ALK, and ROS1 versus next-generation sequencing (NGS) in advanced adenocarcinoma lung cancer patients. BMC Cancer. 2020;20(1). https://doi.org/10.1186/s12885-020-07240-2.
Seo MK, Cairns J. How are we evaluating the cost-effectiveness of companion biomarkers for targeted cancer therapies? A systematic review. BMC Cancer. 2021;21(1):1–21. https://doi.org/10.1186/s12885-021-08725-4.
Tsukumo Y, Suzuki T, Naito M. Current and future issues of companion diagnostics (in Japanese). Regulatory Science of Medical Products. 2017;7(2):71–80.
Doble B, Tan M, Harris A, Lorgelly P. Modeling companion diagnostics in economic evaluations of targeted oncology therapies: systematic review and methodological checklist. Expert Rev Mol Diagn. 2015;15(2):235–54. https://doi.org/10.1586/14737159.2014.929499.
Japanese Society of Medical Oncology the KRAS Mutation Review Subcommittee. Guidance on the measurement of KRAS mutations in patients with colorectal cancer (In Japanese). 2008. https://www.jsmo.or.jp/about/doc/20090128Daichogan.pdf.
Ministry of Health Labour and Welfare in Japan. Medical fee list: appendix table1 (in Japanese). 2020. https://www.mhlw.go.jp/content/12404000/000984041.pdf.
Ministry of Health Labour and Welfare in Japan. Medical fee list: part 3, examinations (division 4, category No. D000-D600) (In Japanese). 2020. https://www.mhlw.go.jp/content/12400000/000603751.pdf.
Ministry of Health Labour and Welfare in Japan. 2011 National Health and Nutrition Examination Survey (in Japanese). 2015. https://www.mhlw.go.jp/bunya/kenkou/eiyou/dl/h23-houkoku.pdf.
Ministry of Health Labour and Welfare in Japan. Ministry of Health, Labor and Welfare notification: no. 60 appendix (in Japanese). 2020. https://www.mhlw.go.jp/bunya/koyou/shougaisha04/dl/kokuji02a.pdf.
Fujimoto S, Watanabe T, Sakamoto A, Yukawa K, Morimoto K. Studies on the physical surface area of japanese part 18 calculation formulas in three stages over all age. Jpn J Hyg. 1968;23(5):443–50. https://doi.org/10.1265/jjh.23.443.
Pharmaceuticals and Medical Devices Agency. Patient drug guide: Erbitux injection 100 mg. 2020. https://www.info.pmda.go.jp/downfiles/guide/ph/380079_4291415A1021_1_01G.pdf.
Pharmaceuticals and Medical Devices Agency. Review report (irinotecan hydrochloride, calcium levofolinate hydrate, fluorouracil). 2013. https://www.pmda.go.jp/drugs/2013/P201300151/800015000_22100AMX02236000_A100_2.pdf.
Van Cutsem E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360(14):1408–17. https://doi.org/10.1056/nejmoa0805019.
Van Cutsem E, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29(15):2011–9. https://doi.org/10.1200/JCO.2010.33.5091.
Seo MK, Cairns J. Do cancer biomarkers make targeted therapies cost-effective? A systematic review in metastatic colorectal cancer. Plos One. 2018;13(9). https://doi.org/10.1371/journal.pone.0204496.
Murray CJL. Global burden of disease Le poids de la morbidite dans le monde quantifying the burden of disease : the technical basis for disability-adjusted life years. Bull World Health Organ. 1994;72(3):429–45.
Murray CJ, Lopez AD. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet. 1997;349(9063):1436–42. https://doi.org/10.1016/S0140-6736(96)07495-8.
Acknowledgements
The authors acknowledge academic and industrial experts from the Systems Med & Pharma subdivision in the System-Information-Simulation division of the Society of Chemical Engineers, Japan (SCEJ).
Funding
Open access funding provided by The University of Tokyo. This research is supported by a Grant-in-Aid for Scientific Research (B) no. 21H01699 from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Consent to Participate
Not applicable.
Research Involving Human Participants and/or Animals
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Okamura, K., Tsuchiya, H., Hamada, R. et al. A Systems Evaluation Model for the Development of Companion Diagnostics and Associated Molecularly Targeted Therapies. J Pharm Innov 18, 2265–2276 (2023). https://doi.org/10.1007/s12247-023-09788-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-023-09788-5