Abstract
Introduction
The development of digital therapies (DTx) has attracted considerable attention in the scientific community worldwide. DTx are software-based therapeutic interventions specifically designed to prevent, manage, or treat various diseases directly in patients.
Method
Given the limited available sources on this topic in the current international scientific literature, the purpose of this review is to provide an overview of the definition and history leading up to the marketing of DTx, as well as to categorize the major DTx currently on the market on the basis of indications and mechanisms of action, with a focus on the regulatory processes that have enabled their use in clinical practice.
Discussion
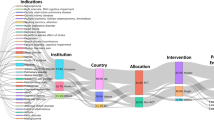
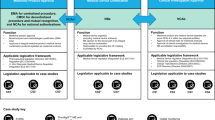
It is well-known that currently regulatory frameworks on DTx are not globally harmonized. The American legislation, for instance, has more uniformly addressed DTx than other legislation such as Europe; in fact, the first globally approved DTX have been authorized in the USA. This lack which currently characterizes regulatory frameworks worldwide may lead to the risk of misclassifying genuine therapies that have undergone multiple clinical trials, each with a distinct impact on patient health.
Conclusion
To obtain full advantage of the enormous potential of DTx, an appropriate regulatory framework needs to be established by the governments of the main countries where DTx are placed on the market, able to complement traditional medicine prescriptions.

Similar content being viewed by others
Data Availability
Full availability of data and materials. All stated data can be provided on request to the reader.
Code Availability
Not applicable.
References
Lavorgna L, et al. E-Health and multiple sclerosis: an update. Mult Scler J. 2018;24. Preprint at: https://doi.org/10.1177/1352458518799629.
Moorhead SA, et al. A new dimension of health care: systematic review of the uses, benefits, and limitations of social media for health communication. J Med Internet Res. 2015;15. Preprint at: https://doi.org/10.2196/jmir.1933.
Kern R, Haase R, Eisele JC, Thomas K, Ziemssen T. Designing an electronic patient management system for multiple sclerosis: building a next generation multiple sclerosis documentation system. Interact J Med Res. 2016;5.
Romagnoli A, Zovi A, Sabbatucci M, Ferrara F, Vitiello A. Therapeutic innovation and digital therapies in the world: comparison between the American and European regulatory framework, with focus on Italy. J Interprof Educ Pract. 2023;100656. https://doi.org/10.1016/j.xjep.2023.100656
DTA. Digital therapeutics alliance. [homepage on the Internet]; 2017. Cited 2020 Jan 09. Available from: https://www.dtxalliance.org/.
Hong JS, Wasden C, Han DH. Introduction of digital therapeutics. Comput Methods Programs Biomed 2021;209 Preprint at https://doi.org/10.1016/j.cmpb.2021.106319.
Chung J-Y. Digital therapeutics and clinical pharmacology. Transl Clin Pharmacol. 2019;27.
Digital therapeutics. Combining technology and evidence-based medicine to transform personalized patient care. Accessed 10 January 2019. https://www.dtxalliance.org/wp-content/uploads/2018/09/DTA-Report_DTx-Industry-Foundations.pdf.
Makin S. The emerging world of digital therapeutics. Nature 2019;573 Preprint at: https://doi.org/10.1038/d41586-019-02873-1.
Mcleroy KR, Bibeau D, Steckler A, Glanz, K. Diabetes Prevention Program (DPP) Research Group. The diabetes prevention program (DPP): description of lifestyle intervention. Am J Prev Med. 41;2020.
United States Patent and Trademark Office. homepage on the internet. 2012. Cited 2020 Jan 09. Available from: http://tsdr.uspto.gov/documentviewer?caseId=sn85765357&docId=FTK20121101070417#docIndex=4&page.
Andrews G, et al. Computer therapy for the anxiety and depression disorders is effective, acceptable and practical health care: an updated meta-analysis. J Anxiety Disord. 2018;55. Preprint at: https://doi.org/10.1016/j.janxdis.2018.01.001.
Beevers CG, et al. Effectiveness of an internet intervention (Deprexis) for depression in a United States adult sample: a parallel-group pragmatic randomized controlled trial. J Consult Clin Psychol. 2017;85.
Morin CM. Profile of Somryst prescription digital therapeutic for chronic insomnia: overview of safety and efficacy. Expert Rev Med Devices. 2020;17 Preprint atL https://doi.org/10.1080/17434440.2020.1852929.
Malgaroli M, Hull TD. Schultebraucks K. Digital health and artificial intelligence for PTSD: improving treatment delivery through personalization. Psychiatr Ann. 2021;51.
Kollins SH, et al. A novel digital intervention for actively reducing severity of paediatric ADHD (STARS-ADHD): A randomised controlled trial. Lancet Digit Health. 2020;2.
Karyotaki E, et al. Efficacy of self-guided internet-based cognitive behavioral therapy in the treatment of depressive symptoms a meta-analysis of individual participant data. JAMA Psych. 2017;74.
Hirschtritt ME. Insel TR. Digital technologies in psychiatry: present and future. Focus (Madison). 2018;16.
Berman MA, et al. Change in glycemic control with use of a digital therapeutic in adults with type 2 diabetes: cohort study. JMIR Diabetes. 2018;20.
Braley M, et al. A virtual, randomized, control trial of a digital therapeutic for speech, language, and cognitive intervention in post-stroke persons with aphasia. Front Neurol. 2021;12.
Everitt H, et al. Assessing cognitive behavioural therapy in irritable bowel (ACTIB): protocol for a randomised controlled trial of clinical-effectiveness and cost-effectiveness of therapist delivered cognitive behavioural therapy and web-based self-management in irritable bowel syndrome in adults. BMJ Open. 2015;5.
Forkmann K, Roth L, Mehl N. Introducing zanadio—a digitalized, multimodal program to treat obesity. Nutrients. 2022;14.
Carl JR, et al. Efficacy of digital cognitive behavioral therapy for moderate-to-severe symptoms of generalized anxiety disorder: a randomized controlled trial. Depress Anxiety. 2020;37.
Espie CA, et al. Effect of digital cognitive behavioral therapy for insomnia on health, psychological well-being, and sleep-related quality of life: a randomized clinical trial. in JAMA Psychiatry. 2019l;79.
Müller-Waldeck, Kalmeda R. Tinnitus. MMW-Fortschritte der Medizin. 2022;164. Preprint at: https://doi.org/10.1007/s15006-022-2016-3.
Benjamens S, Dhunnoo P. Meskó, B. The state of artificial intelligence-based FDA-approved medical devices and algorithms: an online database. NPJ Digit Med. 2020;3.
Kaplan A, Mannarino AP, Nickell PV. Evaluating the impact of Freespira on panic disorder patients’ health outcomes and healthcare costs within the Allegheny Health Network. Appl Psychophysiol Biofeed. 2020;45.
Richter LE, Machleit-Ebner A, Scherbaum N, Bonnet U. How effective is a web-based mental health intervention (Deprexis) in the treatment of moderate and major depressive disorders when started during routine psychiatric inpatient treatment as an adjunct therapy? A pragmatic parallel-group randomized controlled trial. Fortschritte der Neurologie Psychiatrie. 2022. https://doi.org/10.1055/a-1826-2888.
Pöttgen J, et al. Randomised controlled trial of a self-guided online fatigue intervention in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2018;89.
Berger T, et al. Effects of a transdiagnostic unguided Internet intervention ('velibra’) for anxiety disorders in primary care: results of a randomized controlled trial. Psychol Med. 2017;47.
Bergenstal RM, Johnson M, Passi R, Bhargava A, Young N, Kruger DF, Bashan E, Bisgaier SG, Isaman DJM, Hodish I. Automated insulin dosing guidance to optimise insulin management in patients with type 2 diabetes: a multicentre, randomised controlled trial. Lancet. 2019;393(10176):1138–1148. Epub 2019 Feb 23. PMID: 30808512; PMCID: PMC6715130. https://doi.org/10.1016/S0140-6736(19)30368-X.
Chhabra HS, Sharma S, Verma S. Smartphone app in self-management of chronic low back pain: a randomized controlled trial. Eur Spine J. 2018;27.
Iivanainen S, Baird AM, Balas B, Bustillos A, Castro Sanchez AY, Eicher M, Golding S, Mueller-Ohldach M, Reig M, Welslau MAJ. Assessing the impact of digital patient monitoring on health outcomes and healthcare resource usage in addition to the feasibility of its combination with at-home treatment, in participants receiving systemic anticancer treatment in clinical practice: protocol for an interventional, open-label, multicountry platform study (ORIGAMA). BMJ Open. 2023;13(4):e063242.
Lackner JM, et al. Improvement in gastrointestinal symptoms after cognitive behavior therapy for refractory irritable bowel syndrome. Gastroenterology. 2018;155.
Dekker K, et al. Combined Internet-based cognitive-behavioral and chronobiological intervention for insomnia: a randomized controlled trial. Psychother Psychosom. 2020;89 Preprint at: https://doi.org/10.1159/000503570.
Zill JM, et al. Vorvida: study protocol of a randomized controlled trial testing the effectiveness of Internet-based self-help program for the reduction of alcohol consumption for adults. BMC Psychiatry. 2016;16.
Campbell ANC, et al. Internet-delivered treatment for substance abuse: a multisite randomized controlled trial. Am J Psych. 2014;171.
Christensen DR, et al. Adding an internet-delivered treatment to an efficacious treatment package for opioid dependence. J Consult Clin Psychol. 2014;82.
Christensen H, et al. Effectiveness of an online insomnia program (SHUTi) for prevention of depressive episodes (the GoodNight Study): A randomised controlled trial. Lancet Psych 2016;3.
Merchant RK, Inamdar R, Quade RC. Effectiveness of population health management using the propeller health asthma platform: a randomized clinical trial. J Allergy Clin Immunol. 2016;4
Weinstein MM, Pulliam SJ. Richter HE. Randomized trial comparing efficacy of pelvic floor muscle training with a digital therapeutic motion-based device to standard pelvic floor exercises for treatment of stress urinary incontinence (SUV trial): an all-virtual trial design. Contemp Clin Trials. 2021;105.
Denis F. Web-mediated follow-up and prognosis in lung cancer patients. Medecine/Sciences 2018;34.
Richmond S. et al. Digital cognitive training in children with attention-deficit/hyperactivity disorder: a study protocol of a randomised controlled trial. BMJ Open. 2022;12.
Nierenburg H. Stark-Inbar, A. Nerivio®remote electrical neuromodulation for acute treatment of chronic migraine. Pain Manag. 2022;12.
Weise, H. et al. The effect of an app-based home exercise program on self-reported pain intensity in unspecific and degenerative back pain: pragmatic open-label randomized controlled trial. J Med Int Res. 2022;24.
Quinn CC, et al. Cluster-randomized trial of a mobile phone personalized behavioral intervention for blood glucose control. Diabetes Care. 2011;34.
D’Agostino RB, Kwan, H. Measuring effectiveness. What to expect without a randomized control group. Medical Care. 1995;33.
Sverdlov O, van Dam J, Hannesdottir K, Thornton-Wells, T. Digital therapeutics: an integral component of digital innovation in drug development. Clin Pharmacol Ther. 2018;104.
Hung HM J, Wang SJ, Tsong Y, Lawrence J, O’Neil RT. Some fundamental issues with non-inferiority testing in active controlled trials. Stat Med. 2003;22.
Nassir Ghaemi S, Sverdlov O, Van Dam J, Campellone T, Gerwien RA. smartphone-based intervention as an adjunct to standard-of-care treatment for schizophrenia: randomized controlled trial. JMIR Form Res. 2022;6.
Davenport, ND. Werner JK. A randomized sham-controlled clinical trial of a novel wearable intervention for trauma-related nightmares in military veterans. J Clin Sleep Med. 2023;19.
Khozin S, Coravos A. Decentralized trials in the age of real-world evidence and inclusivity in clinical investigations. Clin Pharmacol Ther. 2019;109.
Lubitz SA, et al. Detection of atrial fibrillation in a large population using wearable devices: the Fitbit heart study. Circulation. 2022;146.
Moore TR. A primer of drug action: a concise nontechnical guide to the actions, uses, and side effects of psychoactive drugs, ninth edition. Am J Health-System Pharma. 2002;59.
Lepage, L. et al. The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann Int Med. 2001;134. Preprint at https://doi.org/10.7326/0003-4819-134-8-200104170-00012.
Boucher E, et al. Immediate and long-term effects of an 8-week digital mental health intervention on adults with poorly managed type 2 diabetes: protocol for a randomized controlled trial. JMIR Res Protoc. 2020;9.
Espie CA, et al. A randomized, placebo-controlled trial of online cognitive behavioral therapy for chronic insomnia disorder delivered via an automated media-rich web application. Sleep. 2012;135.
Chaple M, et al. A comparative study of the therapeutic education system for incarcerated substance-abusing offenders. Prison J. 2016;96.
Khirasaria R, Singh V, Batta A. Exploring digital therapeutics: the next paradigm of modern health-care industry. Perspect Clin Res. 2020;11. Preprint at https://doi.org/10.4103/picr.PICR_89_19.
Patel NA, Butte AJ. Characteristics and challenges of the clinical pipeline of digital therapeutics. NPJ Digit Med. 2020;3 Preprint at: https://doi.org/10.1038/s41746-020-00370-8.
Refolo PSDRCSAG. Ethics of digital therapeutics (DTx). Eur Rev Med Pharmacol Sci. 2022;(18):6418–6423. PMID:36196692. https://doi.org/10.26355/eurrev_202209_29741.
Examples of software functions for which the FDA will exercise enforcement discretion [monograph on the internet] USFDA. [Updated 2019 Sept 26; Cited 2020 Jan 09]. Available from: https://www.fda.gov/medical-devices/device-software-functions-includingmobile-medical-applications/examples-software-functions-which-fda-will-exercise-enforcement-discretion.
Examples of mobile apps for which the FDA will exercise enforcement discretion (last updated: August 1st 2016). https://www.fda.gov/MedicalDevices/DigitalHealth/MobileMedicalApplications/ucm368744.
Author information
Authors and Affiliations
Contributions
AR: writing, original draft, and methodology. FV: methodology, and writing, review and editing. AZ: conceptualization, supervision, and validation. AC: writing, review and editing, supervision, and validation. AV: writing, review and editing, supervision, and validation. FF: supervision and validation.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
The authors consent to the publication of the manuscript.
Competing Interests
The authors declare no competing interests.
Disclaimer
The authors declare that the opinions expressed are of a personal nature and do not in any way commit the responsibility of the administrations to which they belong.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Romagnoli, A., Valentino, F., Zovi, A. et al. The Development of Digital Therapies as a New Therapeutic Option to Treat Diseases: Focus on the International Regulatory Framework. J Pharm Innov 18, 2447–2458 (2023). https://doi.org/10.1007/s12247-023-09767-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-023-09767-w