Abstract
Purpose
Liqui-Tablet is a dosage form derived from Liqui-Mass technology. It has proven to be a promising approach to improve drug dissolution rate of poorly water-soluble drugs. So far, Liqui-Tablet is feasible for low-dose drugs. In this study, an attempt was made to produce high-dose Liqui-Tablet, whilst maintaining ideal physicochemical properties for ease of manufacturing.
Methods
Liqui-Tablets containing 100 mg of ketoprofen were produced using various liquid vehicles including PEG 200, Span 80, Kolliphor EL, PG, and Tween 85. Investigations that were carried out included saturation solubility test, dissolution test, tomographic study, and typical quality control tests for assessing flowability, particle size distribution, friability, and tablet hardness.
Results
The weight of these Liqui-Tablets was acceptable for swallowing (483.8 mg), and the saturation solubility test showed PEG 200 to be the most suitable liquid vehicle (493 mg/mL). Tests investigating physicochemical properties such as flowability, particle size distribution, friability, and tablet hardness have shown no issue concerning quality control and manufacturability. The drug release test of the best formulation has shown extremely rapid drug release at pH 7.4 (100% after 5 min). At pH 1.2 the drug release was reasonable considering the formulation was yet to be optimized.
Conclusion
Despite the high amount of API and liquid vehicle, it is possible to produce a high-dose dosage form with acceptable size and weight for swallowing using the novel Liqui-Mass technology. This has the potential to diversify the technology by removing the restriction of high dose drug that has been seen in liquisolid technology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
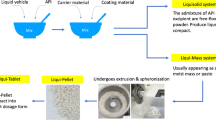
Liqui-Tablet stems from Liqui-Pellet, which is essentially compacted Liqui-Pellet under the Liqui-Mass system. Liqui-Pellet is a combination of concepts from liquisolid technology and pelletization technology [1]. It is different from liquisolid technology in that it uses the Liqui-Mass system instead of the liquisolid system [2]. The Liqui-Mass system is a wet mass system belonging to Liqui-Mass technology, and liquisolid system is free-flowing powder system belonging to liquisolid technology as shown in Fig. 1. Liqui-Mass technology (also known as Liqui-Pellet technology) can produce Liqui-Pellet and Liqui-Tablet, and liquisolid technology typically produces liquisolid compacts.
One of the key purposes of this technology is to improve drug bioavailability through improving its dissolution rate, particularly poorly water-soluble drugs and especially drugs in class II Biopharmaceutics Classification System (BCS) [1, 3,4,5,6]. This is because the bioavailability of such drugs is limited by the absorption, which is limited by the drug dissolution rate [7]. Liqui-Pellet and Liqui-Tablet’s main mechanism of enhanced drug dissolution rate is similar to that of liquisolid formulation. This includes increasing surface area available for dissolution, increased solubility of API, and improved wettability of drug particles, which results in enhanced drug release rate [8, 9]. The early studies on Liqui-Mass technology or Liqui-Pellet technology have shown promising performance in terms of rapid drug release of water insoluble drug such naproxen [10].
Ketoprofen is a weakly acidic nonsteroidal anti-inflammatory drug (NSAID) with analgesic and antipyretic action, which is widely used for rheumatoid arthritis, cancer, and postoperative pain. It is poorly water-soluble and belongs to class II according to the BCS. It works by an inhibitory effect on peripheral COX-1 and COX-2, which in turn reduces the synthesis of prostaglandin and thromboxane precursor [11]. Ketoprofen poor water-solubility makes it a suitable drug candidate for Liqui-Tablet dosage form due to Liqui-Tablet capability in enhancing drug release in an aqueous medium such as the gastro-intestinal fluid.
Compression of pellets into a tablet is a modern technological process and is considered more ideal than enclosing the pellets in a capsule [12, 13]. It is a well-known fact that the tablet dosage form is a more commercially favourable dosage form than a capsule in terms of cost-effectiveness. This is due to lower production cost and higher production rate compared to capsule [14], along with the elimination of control steps that are seen in capsule, i.e. ensuring capsule integrity, which can further increase the cost of production [12, 15]. Also, the tablet dosage form has a lower tendency of dosage form adhering to the oesophagus during ingestion [16], reduces the risk of dosage form being tampered with [14], has the ability to administer higher dose strength than capsule [17], and improves patient compliance, particularly for those who prefer not to ingest gelatine capsule [18]. The issue with gelatine capsule further extends to chemical instability [19], such as varying dissolution rate of capsule due to varying structure and composition of the gelatine [20], and questionable source, particularly from waste leather which has been treated with harmful substance [21]. Therefore, there is a reason to explore the feasibility of high dose Liqui-Tablet.
Advantages of Liqui-Tablet include inherent advantages from Liqui-Pellet dosage form, inherent advantages from liquisolid concept, inherent advantages from pelletization technology, and multi-unit pellet system (MUPS), provided that the Liqui-Tablet reverts back to MUPS in the dissolution medium. The combined array of inherent advantages include a simple method of manufacturing, omission of advance preparation and machinery, cost-effectiveness, capability for green technology, potential for practical upscale of production, the excipients used are common and easily obtainable, good flow property [22], reduction of dust associated problem as seen in tableting powders [23], potential to incorporate incompatible drugs or drugs with different dissolution profiles in same dose unit, flexibility for modification using coating technology, reduced likelihood of side effects due to fast gastric emptying, and improve the predictability drug release as a subunit in MUPS can be distributed more evenly in the GIT [15, 24, 25], which also improves bioavailability [26]. Furthermore, MUPS represents a higher technical barrier to deter the introduction of generic products, hence potentially extending the commercial value of a given drug [26].
In compaction of uncoated pellets, it has been suggested that there are four stages involved. They are (1) rearrangement of pellets, (2) surface deformation, (3) bulk deformation, and (4) cessation of volume reduction [27, 28]. During the low compression force of rearrangement of pellets, the pellets fill the inter-particle void, reducing the volume. At moderate compaction force, the reduction of volume is caused by local surface deformation, where the surface of pellets is flattened. At high compaction force, bulk deformation of pellets occurs, which means that the changes in pellet dimension are in parallel to the densification of pellet. Still under high compaction force, however, there is no further volume reduction due to low inter-granular and intra-granular porosity at the fourth stage [27, 28].
In spite of the array of advantages in pellet-based tablet, which can revert back to MUPS, the manufacturing of such a dosage form is an extremely challenging area of study. The content uniformity of compacted pellets can be influenced by the size of the pellets, pellet size distribution, and the size of additional excipients. In general, compaction of pellets together with excipients of smaller particle size can result in high variation in mass and size due to the segregation phenomenon [29]. The main challenge, however, concerns with coated pellet. The induced damage of functional polymeric coating due to the compression process poses a major issue in a pellet-based tablet. Fortunately, the Liqui-Pellet used in the investigation are not film-coated; therefore, the issue of coating material rupturing is not of concern.
Ideally, the compacted pellets should rapidly revert to MUPS with the same drug release profile as the uncompressed MUPS [26, 27]. The pellet core should be soft enough to deform under the compression force without brittle fracture, but hard enough to resist compression force to prevent permanent fusion of the pellets [26, 27]. In other words, the major mechanism during compaction of pellet should be elastic deformation as oppose to plastic deformation [30].
Since MCC-based pellet is used in this investigation, it is noteworthy to point out some of the observations in studies relating to compression of MCC-base pellet by Johansson et al. [28, 31]. It was observed that MCC-based pellets compressed by deformation and the incidence of pellet fragmentation is very low or non-existence. It is also stated that the MCC-based pellet is inappropriate for enhanced drug release formulation as it does not disintegrate quickly [26]. However, in previous studies on Liqui-Pellet technology [3,4,5, 10], improved dissolution rate can be achieved using Liqui-Mass technology, which contains MCC as the main bulking agent.
In this investigation, the aim is to further explore the potential of Liqui-Tablet (and effectively Liqui-Pellet); to see the feasibility of producing high dose Liqui-Tablet (100 mg ketoprofen) whilst maintaining suitable size and weight for swallowing. The intention is to diversify the technology, allowing high-dose Liqui-Tablet or Liqui-Pellet to be produced in a commercially acceptable manner, which is not possible or very challenging in liquisolid technology.
Materials and Methods
Materials
The API, Ketoprofen, was purchased from Tokyo Chemical Industry Co (TCI, Japan). The carrier in making Liqui-Tablets was microcrystalline cellulose (Avicel PH-102) (FMC corp., UK), which is a purified and partially depolymerized alpha-cellulose. The coating material used was colloidal silicon dioxide (Aerosil 300) (Evonik Industries AG, Hanau, Germany), which is a hydrophilic fumed silica. Other excipients used included Primojel® (sodium starch glycolate Type A), sorbitan laurate (Span 80) (Gattefosse, Saint Priest, France), polyethylene glycol 200 (Fisher Scientific, Leicester, UK), propylene glycol (SAFC, Spain), polysorbate 85 (Tween 85) (Acros, Netherlands), and macrogolglycerol ricinoleate 35 (Kolliphor EL) (BASF, Ludwigshafen, Germany). All other reagents and solvent were of analytical grades.
Saturation Solubility Test
The saturated solubility test of ketoprofen in five different liquid vehicles was performed. Liquid vehicles that were used were as follows: Span 80, polyethylene glycol 200 (PEG 200), propylene glycol (PG), Kolliphor EL, and Tween 85. The preparation of saturated solutions was made by the addition of excess ketoprofen drug powder in a glass vial containing 10 mL of selected liquid vehicle. Each sample of excess ketoprofen in liquid vehicle was then placed in a mechanical bath shaker (OLS Aqua Pro, Grant Instruments Ltd, UK) for 24 h under constant conditions of 37 °C and a shaking speed of 60 rpm. Pre-heated filter (pore size 0.22 µm, Millex GP, Merk Millipore Ltd, Ireland) was used to filter the supernatant, which was then diluted with phosphate buffer solution (pH 7.4). The diluted samples were then analysed spectrophotometrically (Biowave II, Biochrom Ltd, UK) where ketoprofen concentration was determined. Each test was carried out in triplicates.
Preparation of Ketoprofen Liqui-Tablet
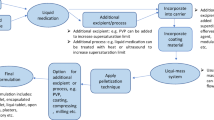
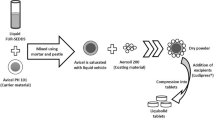
Liqui-Pellets were converted to Liqui-Tablet by compressing them under a compression force of 800 PSI using a manual tablet press machine (Compaction model MTCM-I, Globe pharma, UK). All Liqui-Pellet formulations were produced similarly except for some variables in parameters such as types of non-volatile liquid vehicle and granulating liquid (water) content as shown in Table 1. The liquid medication was made by blending ketoprofen and specified liquid vehicle using pestle and mortar. Carrier material (Avicel PH102) was then incorporated into the liquid medication along with primojel ~ 5% w/w. This superdisintegrant was added intragranularly instead of extragranularly based on previous studies [3].
The admixture was blended using the Caleva Multitab mixing mode setting for 2 min at a constant rate of 125 rpm (Caleva Multitab, Caleva Process Solutions Ltd, UK). A specified amount of deionized water, which was the granulating liquid, was added progressively to achieve suitable mechanical property for extrusion. The wet mass was blended for 5 min before adding coating material (Aerosil 300), which was further blended for another 5 min before extrusion. After extrudates were made, they were spheronized using a rotating friction plate at an almost constant rate of 4000 rpm. The rotation of the friction plate may decrease to 2000 rpm if agglomeration seemed likely. The spheronization time varied depending on the extrudates’ plastic property to avoid agglomeration. An oven set under a constant temperature of 40 °C was then used to removed excess water content overnight.
The physical mixture tablet was prepared similarly as above, but with liquid vehicle omitted. Ratio of the carrier to coating material ratio was kept constant at 20:1 for all formulations. The final weight of all Liqui-Tablet formulations was 483.8 mg, and the physical mixture tablet was 353.8 mg.
Flowability Test on Pre-compressed Formulation
All pre-compressed formulations were subjected to flowability tests. Three key flowability tests were carried out, which included flow rate in g/s, angle of repose, and Carr’s compressibility index. The flowability test that measures the flow rates were conducted by recording the weight (g) and time (s) of the sample flowing through an orifice (10-mm diameter). Note that the shutter was applied before the funnel became completely empty of the specified formulation. The angle of repose was conducted by placing specified Liqui-Pellet formulation in a funnel with an orifice (10 mm in diameter) and letting the sample flow onto a stage. By using a micrometre and digimatic height gauge, measurements of the diameter and height of the heap of the sample were recorded. Such measurement is required to determine the angle of repose using Eq. 1. Carr’s compressibility index (CI%) was calculated from the poured (Pb) and tapped (Pt) densities using CI equation (Eq. 2). Tapped density was determined using the tapped density tester set at 25 taps per min for 4 min. All measurements were done in triplicates.
Particle Size Analysis on Pre-compressed Formulation
Particle size analysis of all pre-compressed formulations was done using sieve method. Weight of 5 g of pre-compressed sample was placed in a sieve (Test sieve, Retsch, Germany) with sizes of 2000, 1000, 850, 500, and 250 µm. These sieves were then placed on a mechanical shaker (AS 200, Retsch, Germany), which vibrated at an amplitude of 60 for a minute before a further vibration of the amplitude of 40 for 9 min. The size distribution of Liqui-Pellet was determined based on pellet fraction between 250 µm and 2000 µm.
Friability Test
A friability test of all formulations was carried out, where ten tablets of each formulation were placed in a friabilator (D-63150, Erweka, Germany), which was set to tumble the tablet sample via rotating for 100 times within 4 min. Samples that have a percentage weight loss of more than 1% or fractured during the tumbling were considered not robust enough and thus fail the test.
Tablet Hardness Test
All formulated tablets were subjected to a tablet hardness test. Each formulation was placed in a tablet hardness tester (TBH 125, Erweka, Germany) where the diameter and thickness of each tablet were measured. The tablet hardness tester then measured the amount of force in N requires to fracture the tablet. This was repeated 5 times for each formulation, and the mean was calculated.
Tomographic Study
The tomographic study was carried out on F-2 as it was the best formulation for rapid drug release. It was carried out using an X-ray microtomography (XμT) (Nikon XT H 225, Nikon Corp. Tokyo, Japan). The instrument was set up using a tungsten target, with 80-μA gun current and 90-kV accelerating voltage. The selected Liqui-Tablet was mounted using double-sided adhesive tape onto a sample stage. Then, a set of 1583 projections was collected from the instrumentation after which these images were reconstructed using CT-Pro, and then examined using VG Studio 2.1 software.
In Vitro Drug Release Test
All formulated tablets were subjected to a dissolution test. The USP paddle method (708-DS Dissolution Apparatus and Cary 60 UV–Vis, Agilent Technologies, USA) was used to carry out the dissolution tests. Each tablet contained 100 mg of ketoprofen. The conditions were set at a temperature of 37.3 ± 0.5 °C, 900mL dissolution medium, and paddle rotation at 50 rpm. Either HCl buffer solution of pH 1.2 or phosphate buffer solution of pH 7.4 was used as dissolution medium to simulate gastric fluid and intestinal fluid in the absence of enzymes. Under the acidic condition, the absorbance readings were taken at 260 nm at time intervals of 5 min for an hour then every 10 min for another hour. Under the alkaline condition, the readings were taken in the same manner as for acidic condition except spectrophotometric absorbance was taken at 262 nm.
Difference factor (f1) equation and similarity factor (f2) as described by Moore and Flanner [32] were the mathematical analysis used for comparing the dissolution profile of different formulations. Both of the mentioned independent-model analysis of drug dissolution rate have been recommended by the US FDA (Food and drug administration) [33] and applied by the FDA in various guidance documents [34, 35]. In brief, f1 value between 0 and 15 and f2 value between 50 and 100 indicates equivalence of the two dissolution profiles [36].
Accelerated Stability Test
Formulation F-2, which had the fastest dissolution rate under pH 1.2, underwent an accelerated stability test. Storage condition was set at 40 °C with a relative humidity of 75% for a period of 3 months. Dissolution profiles were recorded each month for 3 months.
Results
Saturation Solubility Test
The saturation solubility test results are shown in Table 2. According to the test results, it is clear that ketoprofen is most soluble in PEG 200 (~ 493 mg/mL) followed by Tween 85 (~ 295 mg/ml), PG (~ 258 mg/mL), Kolliphor EL (~ 169 mg/mL), then Span 80 (~ 20 mg/mL).
Pre-compression Flowability Test
Flow properties of pre-compressed formulations are shown in Table 3, where according to the angle of repose, all have excellent flowability. In the CI results, the formulations range from excellent to good flowability.
Particle Size Analysis
All pre-compressed Liqui-Tablet formulations generally show narrow size distribution as shown in Fig. 2, where most of the pellets are larger than 500 µm but smaller than 850 µm. The physical mixture pellets are generally larger than the Liqui-Pellets where most pellets are larger than 850 µm but smaller than 2000 µm.
Friability Studies
The friability test results of all formulations are shown in Table 4. All Liqui-Tablet formulations show acceptable robustness as they all passed the friability test. Only the physical mixture tablet failed the friability due to fracturing.
Tablet Hardness Test
The results from the tablet hardness test are shown in Table 5. Note that the physical mixture tablet was too brittle and fractured too easily that the tablet hardness test could not be performed. Different liquid vehicles in Liqui-Tablet show different hardness value. The formulation containing propylene glycol is the hardest (F-3 hardness of 172.6 N) followed by Kolliphor EL (F-4 hardness of 40 N), Tween 85 (F-5 hardness of 34.8 N), Span 80 (F-1 hardness of 25.2 N), then PEG 200 (F-2 hardness of 19.2 N).
Tomographic Studies
X-ray tomography of formulation F-2 (Fig. 3 A1 and B1) shows black spaces, which represents the porosity within the compact. The outline of individual pellets on the surface of the pellet-based tablet can be seen in images A2 and B2 (Fig. 3). The different colourations in A1 and B1 are assigned to the different components in F-2. By looking at the formulation composition (Table 1) and the amount of each excipient used, it is possible to determine which colour belongs to which excipient.
Images from X-ray micro-tomography of F-2 side view (A1 and A2) and top view (B1 and B2). This imaging technique is based on the differential absorbance of X-rays between materials of differing electron density. The colour code is from the density histogram. Based on this, the blue coloration represents the carrier material (Avicel PH-102), the green colouration represents the ketoprofen, and the black coloration represents the pore spaces as well the demarcations of the individual pellets compressed together to form the Liqui-Tablet
In Vitro Dissolution Test
The dissolution profile of all formulations at pH 1.2 and pH 7.4 is shown in Figs. 4 and 5 respectively. It is clear that Liqui-Tablet-enhanced drug release performance is better than the physical mixture tablet. At pH 1.2 (Fig. 4), the formulation containing PEG 200 (F-2) shows the fastest drug release rate, where ~ 92% of the drug is released within 2 h, which is followed by Tween 85 (68% release in 2 h, F-5), Span 80 (62% release in 2 h, F-1), and Kolliphor EL (63% release in 2 h, F-4), then propylene glycol (43% release in 2 h, F-3).
Under alkaline pH of 7.4 (Fig. 5), the dissolution profile of all formulations shows better enhanced drug release than under pH 1.2. However, the order of fastest to slowest drug-releasing formulations are different at pH 7.4 compared to pH 1.2. At pH 7.5, the formulation containing PEG 200 remains to be the fastest (100% release in 5 min, F-2), which is followed by Span 80 (F-1), propylene glycol (F-3), and Tween 85 (F-5) then Kolliphor EL (F-4).
Accelerated Stability Studies
The drug release rate of the best formulation, which contained PEG 200 (F-2), was investigated under stress conditions specified under the accelerated stability test over 3 months (Fig. 6). There seems to be a slight decrease in drug release rate over time, which will be analysed using model-independent analysis and explained in the discussion.
Discussion
Weight of Liqui-Tablet
Despite Liqui-Tablet containing 100 mg of ketoprofen, which is considered a high dose in technology that uses liquid medication in solid carrier (i.e. liquisolid technology), the weight of all formulated Liqui-Tablets is only 483.8 mg. This is considered acceptable for swallowing. In the liquisolid formulation, such a high-dose API would have been near impossible to achieve with ideal flow properties. This is due to a larger amount of liquid vehicle being required to dissolve a sufficient amount of high dose API, which in turn creates an issue in terms of flowability as more liquid will make the admixture wet and cohesive. This would result in formulation requiring an increase of carrier and coating material, rendering the liquisolid compact above 1 g in weight [8]. Unlike liquisolid compact, Liqui-Tablet shows it is capable of high-dose API with acceptable weight, acceptable size, and excellent pre-compression flow properties. With the capability of high-dose drug in Liqui-Tablet, more variety of drugs will be suitable for this novel dosage form.
It should be pointed out that in a study by Espíndola et al. [37], it was claimed that 100 mg ritonavir liquisolid pellets were made. Despite claiming to be liquisolid, the system that was used cannot be considered liquisolid because the admixture was likely none flowable until spheronized. The system used was similar to that of Liqui-Mass system, which is fundamentally different from liquisolid system [2].
Since high-dose drug in Liqui-Tablet does not pose a major issue as it does in liquisolid formulations, there is potential for incorporation of functional excipients, bringing more flexibility and function in formulation design.
Saturation Solubility Test
The results from Table 2 indicate that ketoprofen is most soluble in PEG 200 (freely soluble) and is the most likely suitable liquid vehicle candidate for ketoprofen enhanced drug release Liqui-Tablet. In general, the solubility test results usually correspond to the dissolution test results, which means greater solubility would lead to faster drug release. Although this agrees with the general trend, there are some discrepancies according to dissolution test results in Figs. 4 and 5. Furthermore, the dissolution profile trend differs in acidic and alkaline conditions. Hence, it should be reminded that API solubility is not the only factor that can influence the drug dissolution rate. Other physicochemical characteristics of liquid vehicle such as viscosity, polarity, lipophilicity, chemical structure, and molecular mass may affect drug release [7]. Nevertheless, drug solubility in a liquid vehicle is a major factor that could greatly influence the drug release profile.
Pre-compression Flowability Test and Particle Size Analysis
The results in Table 3 show excellent or good flowability, which is typical of Liqui-Pellets observed in previous studies [1, 3,4,5]. Such data further supports the claim that flow property is not a major issue in Liqui-Pellet or Liqui-Mass technology. This is one of the distinguishing advantages of Liqui-Mass technology over liquisolid technology.
The data from particle size analysis (Fig. 2) shows narrow size distribution for all pre-compressed formulations, which is ideal because it will reduce weight and content variation when filled into a capsule or a hopper for tablet production. The physical mixture pellets seem to have a wider size distribution than the pre-compressed Liqui-Tablet, which suggest that the presence of liquid vehicle influences size distribution. It is noteworthy to state that the size distribution of pellets is rather complex to control. There are numerous factors that can influence pellet size during the extrusion-spheronization process. These factors are API and excipients size [38,39,40,41,42,43], extruder types, properties of extrusion screen, extrusion speed, spheronization speed [44], spheronization time [45,46,47,48], and spheronization load [46, 47, 49].
Friability, Tablet Hardness, and Tomographic Studies
All Liqui-Tablet formulations passed the friability test (Table 4), which is crucial in the perspective of the quality control test involved in manufacturing. The physical mixture tablet, which does not contain liquid vehicle, failed the friability test due to fracturing. Since all formulations were subjected to a compression force of 800 PSI, compression force parameter can be excluded; therefore, it is reasonable to claim that the liquid vehicle is responsible for Liqui-Tablet robustness. It is postulated that the presence of the liquid vehicle in the tablet increases the tablet plasticity and reduces the brittleness; hence, the tablet can absorb impact better without fracturing the tablet during the tumbling in the friabilator.
In terms of the tablet hardness test, it is clear that different liquid vehicles can influence the tablet hardness as presented in Table 5. The data shows that liquid vehicles can have a major impact on Liqui-Tablet hardness, which is shown when comparing the formulation containing propylene glycol (F-3 hardness of 172.6 N) and PEG 200 (F-2 hardness of 19.2 N). Formulation F-3 is around nine times harder than F-2. Since measuring tablet hardness is part of a quality control test, Liqui-Tablets must have acceptable hardness; therefore, the choice of liquid vehicle may become an important factor when considering manufacturability and end product requirement.
The images from the tomographic study (Fig. 3) show the components in the Liqui-Tablet is distributed uniformly and the pellets somewhat retain their multiparticulate form after compressed into a tablet; the pellets not permanently fused; therefore, the tablet produced was able to revert back to individual pellets when in contact with dissolution medium.
In Vitro Dissolution Study
The dissolution profile of all formulations at pH 1.2 is shown in Fig. 4. Formulation F-2 shows the fastest drug release rate, where ~ 92% of the drug is released within 2 h. This is considered fast considering ketoprofen is virtually insoluble in acidic aqueous condition and that the formulation has not yet been optimized, i.e. incorporation of an effervescent agent or optimizing composition ratio.
The next fastest enhanced release formulation is F-5, followed by either F-1 or F-4, then F-3. The dissolution profile of F-1 and F-4 formulations are similar, which is indicated in the difference and similarity factor (f1 = 9.12 and f2 = 67.54). As seen in the saturation solubility test in Table 2, not all dissolution profiles correspond with the solubility test results, reminding that API solubility in the liquid vehicle is not the only factor influencing the drug release rate.
Under alkaline pH of 7.4, the dissolution profile of all formulations generally improves (Fig. 5); however, the hierarchy trend of the drug release rate of various formulations is different from at acidic condition of pH 1.2. This indicates that the effect of liquid vehicle influence on the Liqui-Tablets drug release rate is affected by the environmental pH. Formulation F-2 remains to be the fastest followed by F-1, either F-3 or F-5, then F-4.
The drug release rate of F-2 at pH 7.4 is very rapid, where 100% of the drug is released within 5 min. This is extremely fast in comparison to ketoprofen liquisolid compact. In studies by Vittal et al. [50], 50 mg of ketoprofen liquisolid compact achieved 100% drug release after 20 min under phosphate buffer solution of pH 7.4 at 37 ± 2 °C. The speed of paddle rotation was unspecified. It is clear that Liqui-Tablet drug release rate is by far more superior than liquisolid compact.
Ketoprofen Liqui-Tablet can achieve a rapid drug release rate at pH 7.4 and a reasonable dissolution rate at pH 1.2 when considering the formulation is yet to be optimized. The drug release performance along with acceptable dosage form weight and excellent pre-compressed flow property is ideal for commercial manufacturing.
Accelerated Stability Study Using Dissolution Profile
The dissolution profile of the best formulation containing PEG 200 (F-2) that is shown in Fig. 6 reveals the stability of this Liqui-Tablet over 3 months under accelerated stability test conditions. In comparing F-2 drug dissolution profile at month 0 and a month afterwards (month 1), there is no significant difference according to the model-independent analysis: difference factor of 12.06 and similarity factor of 58.96. A similar observation was observed when comparing F-2 dissolution profile in month 1 and month 2 (f1 = 2.94 and f2 = 83.33). Again the model-independent analysis shows equivalence of dissolution profile for month 2 and month 3 (f1 = 11.14 and f2 = 59.75). However, when comparing the dissolution profile from month 0 and month 3, these dissolution profiles are different (f1 = 24.98.06 and f2 = 45.62). This difference in the drug release profile may be due to possible changes in the formulation over storage time such as Liqui-Tablet became harder over time as a result of further evaporation of water within the tablet. Also, there may be a potential for a small degree of recrystallization of ketoprofen. If that is the case, then the impact of recrystallization on drug dissolution rate is only noticeable over a long period under accelerated stability test condition (3 months under condition setting of 40 °C with a relative humidity of 75%).
Conclusion
The studies show that it is possible to produce high dose Liqui-Tablet (100 mg ketoprofen), whilst maintaining acceptable weight (483.8 mg) and excellent pre-compression flow property. This is a major advancement as it takes liquisolid concept into a commercially feasible direction for high dose drugs, which is impossible or near impossible in the liquisolid technology. Through overcoming the issue of the bulky and heavy weight dosage form of high dose drug in liquisolid formulation, Liqui-Tablet will have a wider range of high-dose API it can be applied to. Not only acceptable weight and excellent pre-compression flow property were observed, but the study also shows ketoprofen Liqui-Tablet to have acceptable robustness and acceptable stability, which is considered ideal in terms of manufacturing and quality control test.
Among the liquid vehicle used in the study, PEG 200 is the most suitable for enhanced drug release of ketoprofen Liqui-Tablet. It is also observed that at different pH, the influence of liquid vehicle on drug release rate changes to a point that some formulations may perform better than others depending on the pH environment.
The formulation containing PEG 200 shows an extremely rapid drug release at pH 7.4 (100% after 5 min), which is much faster than liquisolid compact. At pH 1.2, the drug release is reasonable considering the formulation is yet to be optimized. There is still potential for formulation parameters to be optimized and functional excipients (i.e. effervescent agent) to be incorporated. Furthermore, since Liqui-Tablet is essentially a compressed Liqui-Pellet, the new findings also apply to high dose Liqui-Pellet formulation.
References
Lam M, Ghafourian T, Nokhodchi A. Liqui-pellet: the emerging next-generation oral dosage form which stems from liquisolid concept in combination with pelletization technology. AAPS PharmSciTech. 2019;20(6):1–6.
Lam M, Ghafourian T, Nokhodchi A. Liquisolid system and liqui-mass system are not the same. AAPS PharmSciTech. 2020;21(3):1–3.
Lam M, Ghafourian T, Nokhodchi A. Optimising the release rate of naproxen liqui-pellet: a new technology for emerging novel oral dosage form. Drug Deliv Transl Res. 2020;10(1):43–58.
Lam M, Commandeur D, Maniruzzaman M, Tan DK, Nokhodchi A. The crucial effect of water and co-solvent on Liqui-Pellet pharmaceutical performance. Adv Powder Technol. 2020;31(5):1903–14.
Lam M, Nokhodchi A. Factors affecting performance and manufacturability of naproxen Liqui-Pellet. DARU Journal of Pharmaceutical Sciences. 2020;28(2):567–79.
Lam, M. The making of liqui-pellet and liqui-tablet, the next generation oral dosage form. Unpublished PhD thesis. University of Sussex, Brighton, UK. 2018.
Spireas S, Sadu S. Enhancement of prednisolone dissolution properties using liquisolid compacts. Int J Pharm. 1998;166(2):177–88.
Nokhodchi A, Hentzschel CM, Leopold CS. Drug release from liquisolid systems: speed it up, slow it down. Expert Opin Drug Deliv. 2011;8(2):191–205.
Spireas S, Sadu S, Grover R. In Vitro Release Evaluation of Hydrocortisone Liquisolid Tablets. J Pharm Sci. 1998;87(7):867–72.
Lam M, Asare-Addo K, Nokhodchi A. Rapid releasing naproxen Liqui-Pellet using effervescent agent and neusilin US2. Iran J Basic Med Sci. 2021;24(1):108–15.
Roda A, Sabatini L, Mirasoli M, Baraldini M, Roda E. Bioavailability of a new ketoprofen formulation for once-daily oral administration. Int J Pharm. 2002;241(1):165–72.
Sawicki W, Łunio R. Compressibility of floating pellets with verapamil hydrochloride coated with dispersion Kollicoat SR 30 D. Eur J Pharm Biopharm. 2005;60(1):153–8.
Tunón Å, Börjesson E, Frenning G, Alderborn G. Drug release from reservoir pellets compacted with some excipients of different physical properties. Eur J Pharm Sci. 2003;20(4–5):469–79.
Çelik M. Compaction of multiparticulate oral dosage forms. Drugs and the pharmaceutical sciences. 1994;65:181–215.
Bechgaard H, Nielsen GH. Controlled-release multiple-units and single-unit doses a literature review. Drug Dev Ind Pharm. 1978;4(1):53–67.
Marvola M, Rajaniemi M, Marttila E, Vahervuo K, Sothmann A. Effect of dosage form and formulation factors on the adherence of drugs to the esophagus. J Pharm Sci. 1983;72(9):1034–6.
Zeeshan F, Bukhari NI. Development and evaluation of a novel modified-release pellet-based tablet system for the delivery of loratadine and pseudoephedrine hydrochloride as model drugs. AAPS PharmSciTech. 2010;11(2):910–6.
Bashaiwoldu AB, Podczeck F, Newton JM. The application of non-contact laser profilometry to the determination of permanent structural change induced by compaction of pellets: II. Pellets dried by different techniques. Eur J Pharm Scie 2004;22(1):55–61.
Brown J, Madit N, Cole ET, Wilding IR, Cade D. The effect of cross-linking on the in vivo disintegration of hard gelatin capsules. Pharm Res. 1998;15(7):1026–30.
Hanani ZN, Roos YH, Kerry JP. Use of beef, pork and fish gelatin sources in the manufacture of films and assessment of their composition and mechanical properties. Food Hydrocolloids. 2012;29(1):144–51.
Mokrejs P, Langmaier F, Mladek M, Janacova D, Kolomaznik K, Vasek V. Extraction of collagen and gelatine from meat industry by-products for food and non food uses. Waste Manage Res. 2009;27(1):31–7.
Reynolds AD. A new technique for production of spherical particles. Manuf Chem. 1970;41(6):40.
Conine JW, Hadley HR. Preparation of small solid pharmaceutical spheres. Drug Cosmet Ind. 1970;106(4):38.
Zhu X, Qi X, Wu Z, Zhang Z, Xing J, Li X. Preparation of multiple-unit floating-bioadhesive cooperative minitablets for improving the oral bioavailability of famotidine in rats. Drug Delivery. 2014;21(6):459–66.
Newton JM. Gastric emptying of multi-particulate dosage forms. Int J Pharm. 2010;395(1–2):2–8.
Chen T, Li J, Chen T, Sun CC, Zheng Y. Tablets of multi-unit pellet system for controlled drug delivery. J Control Release. 2017;262:222–31.
Shajahan A, Chandewar V, Jaiswal SB. A flexible technology for modified-release drugs: Multiple-unit pellet system (MUPS). J Control Release. 2010;147:2–16.
Johansson B, Alderborn G. Degree of pellet deformation during compaction and its relationship to the tensile strength of tablets formed of microcrystalline cellulose pellets. Int J Pharm. 1996;132(1–2):207–20.
Beckert TE, Lehmann K, Schmidt PC. Compression of enteric-coated pellets to disintegrating tablets: uniformity of dosage units. Powder Technol. 1998;96(3):248–54.
Wang C, Zhang G, Shah NH, Infeld MH, Malick AW, McGinity JW. Compaction properties of spheronized binary granular mixtures. Drug Dev Ind Pharm. 1995;21(7):753–79.
Johansson B, Wikberg M, Ek R, Alderborn G. Compression behaviour and compactability of microcrystalline cellulose pellets in relationship to their pore structure and mechanical properties. Int J Pharm. 1995;117(1):57–73.
Moore JW, Flanner HH. Mathematical comparison of dissolution profiles. Pharm Technol. 1996;20(6):64–74.
O'hara T, Dunne A, Butler J, Devane J, IVIVR Cooperative Working Group. A review of methods used to compare dissolution profile data. Pharmaceutical Science & Technology Today. 1998;1(5):214–23.
FDA. Guidance for industry dissolution testing of immediate. Evaluation 4, 15–22, 1997.
FDA. Guidance for industry guidance for industry, chemistry, manufacturing, and controls; in vitro dissolution testing and in vivo bioequivalence documentation. US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluatio. In Vitro, 1997.
Adams E, De Maesschalck R, De Spiegeleer B, Vander Heyden Y, Smeyers-Verbeke J, Massart DL. Evaluation of dissolution profiles using principal component analysis. Int J Pharm. 2001;212(1):41–53.
De Espíndola B, Beringhs AO, Sonaglio D, Stulzer HK, Silva MA, Ferraz HG, Pezzini BR. Liquisolid pellets: a pharmaceutical technology strategy to improve the dissolution rate of ritonavir. Saudi Pharmaceutical Journal. 2019;27(5):702–12.
Barrau JP, Bataille B, Duru C, Jacob M, Cassanas G. INTERET DE LA RELATION MOUILLAGE/RENDEMENT GRANULOMETRIQUE EN EXTRUSION/SPHERONISATION: APPLICATION A QUATRE VARIETES DE LACTOSE. Pharm Acta Helv. 1992;67(4):124–8.
Bianchini R, Vecchio C. Oral controlled release optimization of pellets prepared by extrusion-spheronization processing. Farmaco (Societa chimica italiana: 1989). 1989;44(6):645–54.
Fielden KE, Newton JM, Rowe RC. A comparison of the extrusion and spheronization behaviour of wet powder masses processed by a ram extruder and a cylinder extruder. Int J Pharm. 1992;81(2–3):225–33.
Fielden KE, Newton JM, Rowe RC. The influence of lactose particle size on spheronization of extrudate processed by a ram extruder. Int J Pharm. 1992;81(2–3):205–24.
Newton JM, Chow AK, Jeewa KB. The effect of excipient source on spherical granules made by extrusion/spheronization. Pharmaceutical technology. 1993;17(3):166-.
Wan LS, Heng PW, Liew CV. Spheronization conditions on spheroid shape and size. Int J Pharm. 1993;96(1–3):59–65.
Vervaet C, Baert L, Remon JP. Extrusion-spheronisation: a literature review. Int J Pharm. 1995;116(2):131–46.
Malinowski HJ, Smith WE. Use of factorial design to evaluate granulations prepared by spheronization. J Pharm Sci. 1975;64(10):1688–92.
Chariot M, Frances J, Lewis GA, Mathieu D, Luu RP, Stevens HN. A factorial approach to process variables of extrusion-spheronisation of wet powder masses. Drug Dev Ind Pharm. 1987;13(9–11):1639–49.
Hasznos L, Langer I, Gyarmathy M. Some factors influencing pellet characteristics made by an Extrusion/Spheronisation process. Part I.: effects on size characteristics and moisture content decrease of pellets. Drug development and industrial pharmacy. 1992;18(4):409–37.
Ku CC, Joshi YM, Bergum JS, Jain NB. Bead manufacture by extrusion/spheronization—a statistical design for process optimization. Drug Dev Ind Pharm. 1993;19(13):1505–19.
Hellén L, Yliruusi J. Process variables of instant granulator and spheroniser: III. Shape and shape distributions of pellets. International journal of pharmaceutics. 1993;96(1–3):217–23.
Vittal GV, Deveswaran R, Bharath S, Basavaraj BV, Madhavan V. Formulation and characterization of ketoprofen liquisolid compacts by Box-Behnken design. International journal of pharmaceutical investigation. 2012;2(3):150.
Acknowledgements
The author would like to thank Kofi Asare-Addo for his assistance in tomography.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
All authors declare the following in regard to conflicts of interest as they have filed an International (PCT) Patent Application no. PCT/GB2019/052065 on 24 July 2019 and published on 30 January 2020 (International Publication Number WO2020/021254 A1) entitled Pharmaceutical Methods and Compositions (PEL). The authors confirm that there is no significant financial support associated with this publication, which could have influenced its results.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lam, M., Nokhodchi, A. Producing High-Dose Liqui-Tablet (Ketoprofen 100 mg) for Enhanced Drug Release Using Novel Liqui-Mass Technology. J Pharm Innov 17, 778–790 (2022). https://doi.org/10.1007/s12247-021-09561-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-021-09561-6