Abstract
Purpose
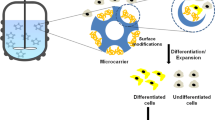
Microcarriers have been extensively explored for culturing anchorage-dependent mammalian cells for various applications. While numerous polymers have been explored for fabricating the microcarriers, both natural and synthetic polymers have their shortcomings. Thus, a synthetic, biocompatible polymer, exhibiting good cell adhesion properties, is the most suitable material for such applications. In this context, polymethylmethacrylate copolymers, commercially available as Eudragit® grades, were investigated for their suitability as matrices for mammalian cell culture.
Methods
Eudragit® RL100 grade was successfully used to fabricate uniform, spherical, and non-porous microcarriers for the first time, having a diameter of 222 ± 60 µm, by the modified emulsion cross-linking/solvent evaporation process. Further, the recombinant cell line CHO 6E6, reported to produce human IgG, was used for evaluating the potential of coated and non-coated microcarriers to support in vitro cell growth and proliferation.
Results
It was observed that cells did not grow on uncoated microcarriers, though some cellular attachment was observed. However, they could attach, grow, and produce IgG on gelatin- and collagen-coated Eudragit® RL100 microcarriers. Microcarriers coated with collagen promoted higher proliferation of cells than those coated with gelatin, while the cells growing on the latter resulted in higher production of IgG.
Conclusion
Functionally active cells could be successfully grown on Eudragit® RL100 microcarriers coated with collagen and gelatin. Gelatin offered a more suitable chemistry for the growth of morphologically appropriate cells, which ultimately lead to higher productivity. While this study is one of the few proof-of-concept investigations to evaluate the potential of Eudragit® microcarriers for mammalian cell culture, further investigations are warranted to demonstrate a match in the productivity of the growing cells with those being cultivated on commercially available microcarriers.









Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author if required. All data generated or analyzed during this study are included in this published article.
References
Harrison RG, Greenman M, Mall FP, Jackson C. Observations of the living developing nerve fiber. Anat Rec. 1907;1(5):116–28. https://doi.org/10.3181/00379727-4-98.
Leong W, Wang D-A. Cell-laden polymeric microspheres for biomedical applications. Trends Biotechnol. 2015;33(11):653–66. https://doi.org/10.1016/j.tibtech.2015.09.003.
Van Wezel A. Growth of cell-strains and primary cells on micro-carriers in homogeneous culture. Nature. 1967;216(5110):64.
Tan WH, Takeuchi S. Monodisperse alginate hydrogel microbeads for cell encapsulation. Adv Mater. 2007;19(18):2696–701. https://doi.org/10.1002/adma.200700433.
Dvir-Ginzberg M, Gamlieli-Bonshtein I, Agbaria R, Cohen S. Liver tissue engineering within alginate scaffolds: effects of cell-seeding density on hepatocyte viability, morphology, and function. Tissue Eng. 2003;9(4):757–66. https://doi.org/10.1089/107632703768247430.
Maeng Y-J, Choi S-W, Kim HO, Kim J-H. Culture of human mesenchymal stem cells using electrosprayed porous chitosan microbeads. J Biomed Mater Res A. 2009;9999A:NA-NA. https://doi.org/10.1002/jbm.a.32417.
Cai H, Sharma S, Liu W, Mu W, Liu W, Zhang X, et al. Aerogel microspheres from natural cellulose nanofibrils and their application as cell culture scaffold. Biomacromol. 2014;15(7):2540–7. https://doi.org/10.1021/bm5003976.
Mooney DJ, Sano K, Kaufmann PM, Majahod K, Schloo B, Vacanti JP, et al. Long-term engraftment of hepatocytes transplanted on biodegradable polymer sponges. J Biomed Mater Res. 1997;37(3):413–20. https://doi.org/10.1002/(SICI)1097-4636(19971205)37:3%3c413::AID-JBM12%3e3.0.CO;2-C.
Gabler F, Frauenschuh S, Ringe J, Brochhausen C, Götz P, Kirkpatrick CJ, et al. Emulsion-based synthesis of PLGA-microspheres for the in vitro expansion of porcine chondrocytes. Biomol Eng. 2007;24(5):515–20. https://doi.org/10.1016/j.bioeng.2007.08.013.
McDevitt TC, Woodhouse KA, Hauschka SD, Murry CE, Stayton PS. Spatially organized layers of cardiomyocytes on biodegradable polyurethane films for myocardial repair. J Biomed Mater Res A. 2003;66(3):586–95. https://doi.org/10.1002/jbm.a.10504.
Carletti E, Motta A, Migliaresi C. Scaffolds for tissue engineering and 3D cell culture. 3D cell culture. Springer; 2011. p. 17–39.
Hong Y, Gao C, Xie Y, Gong Y, Shen J. Collagen-coated polylactide microspheres as chondrocyte microcarriers. Biomaterials. 2005;26(32):6305–13. https://doi.org/10.1016/j.biomaterials.2005.03.038.
Jorge SD, editor. Expansion of multipotent mesenchymal stromal cells on gelatin coated alginate microcarriers. Tecnico Lisboa; 2014; Lisboa, Portugal.
Phillips BW, Horne R, Lay TS, Rust WL, Teck TT, Crook JM. Attachment and growth of human embryonic stem cells on microcarriers. J Biotechnol. 2008;138(1–2):24–32. https://doi.org/10.1016/j.jbiotec.2008.07.1997.
Thakral S, Thakral NK, Majumdar DKJEoodd. Eudragit®: a technology evaluation. Expert Opin Drug Deliv 2013;10(1):131–49. https://doi.org/10.1517/17425247.2013.736962.
Pezzini BR, Schucko SK, Schmücker IC, Zétola M, Wagner TM, Erzinger GS, et al. Preparation and characterization of microparticles containing simvastatin solid dispersions in Eudragit E 100 and poly(3-hydroxybutyrate). J Disper Sci Technol. 2013;34(11):1603–8. https://doi.org/10.1080/01932691.2012.681998.
Joshi AS, Patil CC, Shiralashetti SS, Kalyane NV. Design, characterization and evaluation of Eudragit microspheres containing glipizide. Drug Invent. 2013;5(3):229–34. https://doi.org/10.1016/j.dit.2013.06.009.
Shinde UA, Shete JN, Nair HA, Singh KH. Eudragit RL100 based microspheres for ocular administration of azelastine hydrochloride. J Microencapsul. 2012;29(6):511–9. https://doi.org/10.3109/02652048.2012.665088.
Esposito E, Cervellati F, Menegatti E, Nastruzzi C, Cortesi R. Spray dried Eudragit microparticles as encapsulation devices for vitamin C. Int J Pharm. 2002;242(1–2):329–34. https://doi.org/10.1016/S0378-5173(02)00176-X.
Basu S, Adhiyaman RJTJoPR. Preparation and characterization of nitrendipine-loaded Eudragit RL 100 microspheres prepared by an emulsion-solvent evaporation method. Trop J Pharm Res. 2008;7(3):1033–41. https://doi.org/10.4314/tjpr.v7i3.14688.
Madhusudhan S, Panda AK, Parimalakrishnan S, Manavalan R, Manna PK. Design, in vitroandin vivoevaluation of glipizide Eudragit microparticles. J Microencapsul. 2010;27(4):281–91. https://doi.org/10.3109/02652040903131319.
Kim W-D, Tokunaga M, Ozaki H, Ishibashi T, Honda K, Kajiura H et al. Glycosylation pattern of humanized IgG-like bispecific antibody produced by recombinant CHO cells. Appl Microbiol Biotechnol. 2009;85(3):535–42. https://doi.org/10.1007/s00253-009-2152-z.
Freitas S, Merkle HP. Gander BJJocr. Microencapsulation by solvent extraction/evaporation: reviewing the state of the art of microsphere preparation process technology. 2005;102(2):313–32. https://doi.org/10.1016/j.jconrel.2004.10.015.
Hoa LTM, Chi NT, Nguyen LH, Chien DM. Preparation and characterisation of nanoparticles containing ketoprofen and acrylic polymers prepared by emulsion solvent evaporation method. J Exp Nanosci. 2011;7(2):189–97. https://doi.org/10.1080/17458080.2010.515247.
Ramachandran S, Nandhakumar S, Dhanaraju MD. Formulation and characterization of glutaraldehyde cross-linked chitosan biodegradable microspheres loaded with famotidine. Trop J Pharm Res. 2011;10(3). https://doi.org/10.4314/tjpr.v10i3.13.
Bhumkar DR, Pokharkar VBJAP. Studies on effect of pH on cross-linking of chitosan with sodium tripolyphosphate: a technical note. AAPS PharmSciTech. 2006;7(2):E138–E43. https://doi.org/10.1208/pt070250.
Damink LO, Dijkstra PJ, Van Luyn M, Van Wachem P, Nieuwenhuis P, Feijen J. Glutaraldehyde as a crosslinking agent for collagen-based biomaterials. J Mater Sci Mater Med. 1995;6(8):460–72. https://doi.org/10.1007/BF00123371.
Arifin MA, Mel M, Samsudin N, Hashim YZH-Y, Salleh HM, Sopyan I et al. Ultraviolet/ozone treated polystyrene microcarriers for animal cell culture. J Chem Technol Biotechnol. 2016;91(10):2607–19. https://doi.org/10.1002/jctb.4855.
Khalili A, Ahmad M. A review of cell adhesion studies for biomedical and biological applications. Int J Mol Sci. 2015;16(8):18149–84. https://doi.org/10.3390/ijms160818149.
Solohill S-A. Sigma-Solohill Microcarrier Beads. Prod Information. 2007. p. 1–2.
Acknowledgments
This work was supported by the Department of Biotechnology, Government of India. The authors would like to thank Evonik Healthcare for generously providing the gift sample for Eudragit®. We also want to thank Nano-medicine Research Group for the guidance and support at various stages.
Funding
This work was supported by the Department of Biotechnology, Government of India. Also, Evonik Healthcare generously provided the gift sample for Eudragit® for experimentation.
Author information
Authors and Affiliations
Contributions
Conceptualization: Prajakta Dandekar, Ratnesh Jain; Methodology: Prajakta Dandekar, Nikita Aware, Tejal Pant; Formal analysis and investigation: Nikita Aware, Tejal Pant; Writing - original draft preparation: Nikita Aware; Writing - review and editing: Tejal Pant, Prajakta Dandekar; Resources and Supervision: Prajakta Dandekar, Ratnesh Jain. Aware Nikita carried out the fabrication and modification of the microcarriers, cell culture and characterisation of the cultured cells. Pant Tejal provided guidance for performing cell culture and carried out analysis on the confocal laser scanning microscope. Jain Ratnesh and Dandekar Prajakta conceived the concept for this investigation and participated in the design of the study. All the authors helped to draft and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aware, N., Pant, T., Jain, R. et al. Polymethylmethacrylate Copolymer-Based Microcarriers for Culturing Mammalian Cells. J Pharm Innov 17, 881–891 (2022). https://doi.org/10.1007/s12247-020-09532-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-020-09532-3