Abstract
Purpose
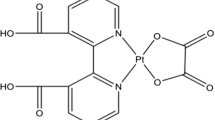
In this work, a new water-soluble Pt(II) complex was synthesized with aliphatic glycine ligand with a formula of cis-[Pt(NH3)2(isopentylgly)]NO3, as an anti-cancer drug, and characterized. To determine the binding constant of the human serum albumin (HSA, the most abundant carrier proteins in the human circulatory system) to this complex and the binding site of the complex on HSA, the melting point of HSA and the kinetics of this interaction were investigated to introduce an anti-breast cancer drug with fewer side effects.
Methods
HSA interaction with the complex was studied via a spectroscopic method at 27 and 37 °C and physiological situation (I = 10 mM, pH = 7.4) and molecular docking.
Results
The toxicity value of this complex was obtained against the human cancer breast cell line of MCF-7. The thermodynamic parameters of enthalpy and entropy were also achieved in the empirical procedure. Due to the spontaneity of the interaction, Gibbs free energy variation was obtained negative. The binding constant of this complex to HSA was 3.9 × 105 (M−1). Empirical results showed that the quenching mechanism was static. Hill coefficients, Hill constant, complex aggregation number around protein, number of binding sites, and protein melting temperature with complex were obtained. The kinetics of this interaction was also investigated, which showed that this interaction follows a second-order kinetic. The molecular docking data indicated that the position of the interaction of complex on the protein was the site I in the sub-second IIA. Also, the hydrogen bonding and the hydrophobic interaction as the dominant binding forces were seen in complex–HSA formation.
Conclusion
This interaction with positive cooperativity was recognized via a superior hydrogen bond. The reasonable binding constant was also obtained, which could ultimately be a good option as an anti–breast cancer drug.


















Similar content being viewed by others
Abbreviations
- HSA:
-
Human serum albumin
- PDB:
-
Protein Data Bank
References
Kazemi Z, Rudbari HA, Sahihi M, Mirkhani V, Moghadam M, Tangestaninejad S, et al. Synthesis, characterization and biological application of four novel metal-Schiff base complexes derived from allylamine and their interactions with human serum albumin: experimental, molecular docking, and ONIOM computational study. J Photochem Photobiol B. 2016;162:448–62. https://doi.org/10.1016/j.jphotobiol.2016.07.003.
Hoang H, Manyanga F, Morakinyo MK, Pinkert V, Sarwary F, Fish DJ, et al. Effects of selective biotinylation on the thermodynamic stability of human serum albumin. J Biophys Chem. 2016;7(1):9–29. https://doi.org/10.4236/ibpc.2016.71002.
Topală T, Bodoki A, Oprean L, Oprean R. Bovine serum albumin interactions with metal complexes. Clujul Med. 2014;87:215–9. https://doi.org/10.15386/cjmed-357.
Zhu M, Cui X, Zhang S, Liu L, Han Z, Gao ZE. The structures, cytotoxicity, apoptosis and molecular docking controlled by the aliphatic chain of palladium (II) complexes. J Inorg Biochem. 2016;157:34–45. https://doi.org/10.1016/j.jinorgbio.2016.01.016.
Kantoury M, Eslami Moghadam M, Tarlani AA, Divsalar A. Structure effect of some new anticancer Pt (II) complexes of amino acid derivatives with small branched or linear hydrocarbon chains on their DNA interaction. Chem Boil Drug Dis. 2016;88(1):76–87. https://doi.org/10.1111/cbdd.12735.
Hadian Rasanani S, Eslami Moghadam M, Soleimani E, Divsalar A, Ajloo A, Tarlani A, et al. Anticancer activity of new imidazole derivative of 1R, 2R-diaminocyclohexane palladium and platinum complexes as DNA fluorescent probes. J Biomol Struct Dyn. 2018;36(12):3058–76. https://doi.org/10.1080/07391102.2017.1385538.
Aghaee E, Ghasemi JB, Manouchehri F, Balalaie S. Combined docking, molecular dynamics simulations and spectroscopic studies for the rational design of a dipeptide ligand for affinity chromatography separation of human serum albumin. J Mol Model. 2014;20(10):2446. https://doi.org/10.1007/s00894-014-2446-7.
Ajmal MR, Zaidi N, Alam P, Nusrat S, Siddiqi MK, Badr G, et al. Insight into the interaction of antitubercular and anticancer compound clofazimine with human serum albumin: spectroscopy and molecular modeling. J Biomol Struct Dyn. 2017;35(1):46–57. https://doi.org/10.1080/07391102.2015.1132258.
Shamsi A, Ahmed A, Bano B. Probing the interaction of anticancer drug temsirolimus with human serum albumin: molecular docking and spectroscopic insight. J Biomol Struct Dyn. 2018;36:1479–89. https://doi.org/10.1080/07391102,2017,1326320.
Shen F, Liu YX, Li SM, Jiang CK, Wang BF, Xiong YH, et al. Synthesis, crystal structures, molecular docking and in vitro cytotoxicity studies of two new copper (ii) complexes: special emphasis on their binding to HSA. New J Chem. 2017;41(21):12429–41. https://doi.org/10.1039/c7nj02351k.
Yasmeen S. Exploring thermodynamic parameters and the binding energetics of berberine chloride to bovine serum albumin (BSA): spectroscopy, isothermal titration calorimetry, and molecular docking techniques. Thermochim Acta. 2017;655:76–86. https://doi.org/10.1016/j.tca.2017.06.010.
Kong L, Hu J, Qin D, Yan P. Interaction of ifosfamide-loaded superparamagnetic iron oxide nanoparticles with human serum albumin—a biophysical study. J Pharm Innov. 2015;10(1):13–20. https://doi.org/10.1007/s12247-014-9199-9.
Ojha H, Mishra K, Hassan MI, Chaudhury NK. Spectroscopic and isothermal titration calorimetry studies of binding interaction of ferulic acid with bovine serum albumin. Thermochim Acta. 2012;2012(548):56–64. https://doi.org/10.1016/j.tca.2012.08.016.
Trynda-Lemiesz L, Wiglusz K. Effects of glycation on meloxicam binding to human serum albumin. J Mol Struct. 2011;995(1–3):35–40. https://doi.org/10.1016/j.molstruc.2011.03.037.
Tang J, Lian N, Bi C, Li W. Analysis of eupatilin-human serum albumin interactions by means of spectroscopic and computational modeling. J Pharm Pharmacol. 2007;59(5):637–43. https://doi.org/10.1211/jpp.59.5.0003.
Mohammadgholi A, Leilabadi-Asl A, Divsalar A, Eslami Moghadam M. Multi-spectroscopic studies of the interaction of new synthesized platin complex with human carrier protein of serum albumin. J Biomol Struct Dyn. 2020:1–6. https://doi.org/10.1080/07391102.2020.1745690.
Kabir MZ, Tee WV, Mohamad SB, Alias Z, Tayyab S. Tayyab, interaction of an anticancer drug, gefitinib with human serum albumin: insights from fluorescence spectroscopy and computational modeling analysis. RSC Adv. 2016;6(94):91756–67. https://doi.org/10.1039/C6RA12019A.
Kandagal PB, Ashoka S, Seetharamappa J, Shaikh SMT, Jadegoud Y, Ijare OB. Study of the interaction of an anticancer drug with human and bovine serum albumin: spectroscopic approach. J Pharm Biomed. 2006;41:393–9. https://doi.org/10.1016/j.jpba.2005.11.037.
Abyaneh FSS, Moghadam ME, Divsalar A, Ajloo D, Sadr MH. Improving anticancer activity and solubility of cisplatin by methyl glycine and methylamine ligands against human breast adenocarcinoma cell line. Appl Biochem Biotechnol. 2018;186(2):271–91. https://doi.org/10.1007/s12010-018-2715-5.
Hadian Rasanani S, Eslami Moghadam M, Soleimani E, Divsalar A, Tarlani A. Improving the activity of anticancer oxalipalladium analog by the modification of oxalate group with isopentylglycine. J Coord Chem. 2017;70(22):3769–89. https://doi.org/10.1080/00958972.2017.1395417.
Becke AD. Density-functional thermochemistry: the role of extract exchange. J Chem Phys. 1993;98:5648–52. https://doi.org/10.1063/1.464913.
Francl MM, Pietro WJ, Hehre WJ, Binkley JS, Gordon MS, DeFrees DJ, et al. Self-consistent molecular orbital methods. XXIII. A polarization-type basis set for second-row elements. J Chem Phys. 1982;77(7):3654–65. https://doi.org/10.1063/1.444267.
Bursulaya BD, Totrov M, Abagyan R, Brooks CL. Comparative study of several algorithms for flexible ligand docking. J Comput Aid Mol Des. 2003;17(11):755–63. https://doi.org/10.1023/b:jcam.0000017496.76572.6f.
Heydari A, Mansouri-Torshizi H. Design, synthesis, characterization, cytotoxicity, molecular docking and analysis of binding interactions of novel acetylacetonatopalladium (II) alanine and valine complexes with CT-DNA and BSA. RSC Adv. 2016;6(98):96121–37. https://doi.org/10.1039/C6RA18803F.
Fu XB, Liu DD, Lin Y, Hu W, Mao ZW, Le XY. Water-soluble DNA minor groove binders as potential chemotherapeutic agents: synthesis, characterization, DNA binding and cleavage, antioxidation, cytotoxicity and HSA interactions. Dalton T. 2014;43(23):8721–37. https://doi.org/10.1039/c3dt53577k.
Bordbar AK, Saadati Z, Sohrabi N. Analysis of ligand binding process using the binding capacity concept. Acta Biochim Pol. 2004;51(4):963–70 10/045104963.
Hosseinzadeh R, Bordbar AK, Matin AA, Maleki R. Potentiometric study of cetylpyridinium chloride cooperative binding to anionic azo-dyes. J Braz Chem Soc. 2007;18(2):359–63. https://doi.org/10.1590/S0103-50532007000200017.
Heydari M, Moghadam ME, Tarlani A, Farhangian H. DNA as a target for anticancer phen-imidazole Pd (II) complexes. Appl Biochem Biotechnol. 2017;182(1):110–27. https://doi.org/10.1007/s12010-016-2314-2.
Bordbar AK, Moosavi-Movahedi AA, Amini MK. A microcalorimetry and binding study on the interaction of dodecyl trimethylammonium bromide with wigeon hemoglobin. Thermochim Acta. 2003;400(1–2):95–100. https://doi.org/10.1016/S0040-6031(02)00483-5.
Proetto MT, Liu W, Molchanov A, Sheldrick WS, Hagenbach AA, Abram U, et al. Synthesis, characterization, and in vitro antiproliferative activity of [salophene] platinum (II) complexes. Chem Med Chem. 2014;9(6):1176–87. https://doi.org/10.1002/cmdc.201402123.
Liu P, Liu J, Zhang YQ, Wu BY, Wang KZ. Synthesis, DNA binding and photocleavage, and cellular uptake of an alkyl chain-linked dinuclear ruthenium (II) complex. J Photochem Photobiol B. 2015;143:89–99. https://doi.org/10.1016/j.jphotobiol.2015.01.004.
Chatterjee T, Pal A, Dey S, Chatterjee BK, Chakrabarti P. Interaction of virstatin with human serum albumin: spectroscopic analysis and molecular modeling. PLoS One. 2012;7(5):e37468. https://doi.org/10.1371/journal.pone.0037468.
Gao D, Tian Y, Bi S, Chen Y, Yu A, Zhang H. Studies on the interaction of colloidal gold and serum albumins by spectral methods. Spectrochim Acta A. 2005;62(4–5):1203–8. https://doi.org/10.1016/j.saa.2005.04.026.
Gupta RK, Kumar A, Paitandi RP, Singh RS, Mukhopadhyay S, Verma SP, et al. Heteroleptic arene Ru (II) dipyrrinato complexes: DNA, protein binding and anti-cancer activity against the ACHN cancer cell line. Dalton S. 2016;45(16):7163–77. https://doi.org/10.1039/C6DT00446F.
Villarreal W, Colina-Vegas L, Rodrigues de Oliveira C, Tenorio JC, Ellena J, Gozzo FC, et al. Chiral platinum (II) complexes featuring phosphine and chloroquine ligands as cytotoxic and monofunctional DNA-binding agents. Inorg Chem. 2015;54:11709–20. https://doi.org/10.1021/acs.inorgchem.5b01647.
Divsalar A, Razmi M, Saboury AA, Mansouri-Torshizi H, Ahmad F. Biological evaluation of a new synthesized Pt (II) complex by cytotoxic and spectroscopic studies. Cell Biochem Biophys. 2015;71(3):1415–24. https://doi.org/10.1007/s12013-014-0364-z.
Shao J, Bao WG, Tian H, Li B, Zhao XF, Qiao X, et al. Nuclease activity and protein-binding properties of a novel tetranuclear thiosemicarbazide Pt (II) complex. Dalton T. 2014;43(4):1663–71. https://doi.org/10.1039/c3dt52044g.
Azimi O, Emami Z, Salari H, Chamani J. Probing the interaction of human serum albumin with norfloxacin in the presence of high-frequency electromagnetic fields: fluorescence spectroscopy and circular dichroism investigations. Molecules. 2011;16(12):9792–818. https://doi.org/10.3390/molecules16129792.
Guha S, Rawat SS, Chattopadhyay A, Bhattacharyya B. Tubulin conformation and dynamics: a red edge excitation shift study. Biochemistry. 1996;35(41):13426–33. https://doi.org/10.1021/bi961251g.
Jahanban-Esfahlan A, Panahi-Azar V, Sajedi S. Spectroscopic and molecular docking studies on the interaction between N-acetyl cysteine and bovine serum albumin. Biopolymers. 2015;103:638–45. https://doi.org/10.1002/bip.22697.
Zhu M, Wang L, Zhang H, Fan S, Wang Z, Li QX, et al. Interactions between tetrahydroisoindoline-1, 3-dione derivatives and human serum albumin via multiple spectroscopy techniques. Environ Sci Pollut Res. 2018;25(2018):17735–48. https://doi.org/10.1007/s11356-018-1955-9.
Mansouri-Torshizi H, Khosravi F, Ghahghaei A, Shahraki S, Zareian-Jahromi S. Investigation on the interaction of newly designed potential antibacterial Zn (II) complexes with CT-DNA and HAS. J Biomol Struct Dyn. 2018;36(10):2713–37. https://doi.org/10.1080/07391102.2017.1363086.
Xiao Y, Xu K, Wang Q, Xiong X, Huang Y, Li H. Synthesis, structure, and calf-thymus DNA binding of ternary fleroxacin–Cu (ii) complexes. RSC Adv. 2016;6(83):80286–95. https://doi.org/10.1039/C6RA18971G.
Esteghamat-Panah R, Farrokhpour H, Hadadzadeh H, Abyar F, Rudbari HA. An experimental and quantum chemical study on the non-covalent interactions of a cyclometallated Rh (III) complex with DNA and BSA. RSC Adv. 2016;6:23913–29. https://doi.org/10.1039/C5RA24540K.
Asadi Z, Mosallaei H, Sedaghat M, Yousefi R. Competitive binding affinity of two lanthanum (III) macrocycle complexes toward DNA and bovine serum albumin in water. J Iran Chem Soc. 2017;14(11):2367–85. https://doi.org/10.1007/s13738-017-1172-3.
Anjomshoa M, Torkzadeh-Mahani M, Sahihi M, Rizzoli C, Ansari M, Janczak J, et al. Tris-chelated complexes of nickel (II) with bipyridine derivatives: DNA binding and cleavage, BSA binding, molecular docking, and cytotoxicity. J Biomol Struct Dyn. 2019;37(15):3887–904. https://doi.org/10.1080/07391102.2018.1534700.
Ghosh P, Devi GP, Priya R, Amrita A, Sivaramakrishna A, Babu S, et al. Spectroscopic and in silico evaluation of the interaction of DNA with six anthraquinone derivatives. Appl Biochem Biotechnol. 2013;170:1127–37. https://doi.org/10.1007/s12010-013-0259-2.
Acknowledgments
The authors of this article would like to thank the Payame Noor University of Isfahan.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shiekhzadeh, A., Sohrabi, N., Eslami Moghadam, M. et al. Effect of Presence of Aliphatic Glycine in the Anti-cancer Platinum Complex Structure on Human Serum Albumin Binding. J Pharm Innov 17, 353–365 (2022). https://doi.org/10.1007/s12247-020-09508-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-020-09508-3