Abstract
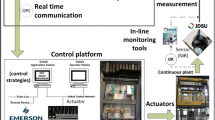
In continuous pharmaceutical manufacturing, real-time precise control of critical quality attributes (CQAs) is necessary for quality by design (QbD)-based manufacturing and real-time release (RTR) with minimum consumption of time, space, and resources. Pharmaceutical tablets can be produced through different routes with a common tablet press unit operation always placed at the end of the manufacturing process. Therefore, the tablet press is a crucial unit operation directly influencing the CQAs irrespective of manufacturing routes. Despite this, little attention has been paid to the development of an advanced efficient control system for the tablet press. Process modeling can be used as an efficient virtual experimentation tool to design, compare, and evaluate different control systems. We developed a model in Simulink (Mathworks) that includes two master control loops for tablet weight and hardness and a slave feedback loop controlling the compaction force applied to each tablet. We examined the performance of different control strategies based on proportional integral derivative (PID) control and model predictive control (MPC), as well as feedforward/feedback control. We found that a hybrid MPC-PID control strategy outperforms the PID-only control strategy. We also observed that the addition of a feedforward controller further improves the performance of the hybrid MPC-PID control strategy.










Similar content being viewed by others
Abbreviations
- Symbols:
-
Variables units
- H:
-
Height (m)
- m:
-
Mass (kg)
- MCF:
-
Main compression force (kN)
- RMSE:
-
Root mean square error (kp, g, kN)
- ISE:
-
Integral of square error (kp2 s, g2 s, kN2 s)
- IAE:
-
Integral of absolute error (kp s, g s, kN s)
- ITAE:
-
Integral of time absolute error (kp s2, g s2, kN s2)
- T2P:
-
Time to product (s)
- M2P:
-
Magnitude to product (kp, g, kN)
- D2R:
-
Disturbance to reject (s)
- P:
-
Proportional constant (mm/kN, kN/g)
- I:
-
Integral constant (s)
- D:
-
Derivative constant (s)
- API:
-
Active pharmaceutical ingredient
- CQAs:
-
Critical quality attributes
- LTI:
-
Linear time invariant
- OE:
-
Output error
- PAT:
-
Process analytical technology
- PID:
-
Proportional integral derivative
- QbD:
-
Quality by design
- MPC:
-
Model predictive control
References
United States Food and Drug Administration. PAT—a framework for innovative pharmaceutical development, manufacturing, and quality assurance. Rockville, Maryland: Food and Drug Administration; 2004. p. 1–16.
Igne B, de Juan A, Jaumot J, Lallemand J, Preys S, Drennen JK, et al. Modeling strategies for pharmaceutical blend monitoring and end-point determination by near-infrared spectroscopy. Int J Pharm. 2014;473(1):219–31.
Valencia-Palomo G, Rossiter J. Programmable logic controller implementation of an auto-tuned predictive control based on minimal plant information. ISA Trans. 2011;50(1):92–100.
Åström KJ, Hägglund T, Hang CC, Ho WK. Automatic tuning and adaptation for PID controllers-a survey. Control Eng Pract. 1993;1(4):699–714.
Richalet J. Industrial applications of model based predictive control. Automatica. 1993;29(5):1251–74.
Marlin TE. Process control. New York: McGraw-Hill; 2000.
Garcia CE, Prett DM, Morari M. Model predictive control: theory and practice—a survey. Automatica. 1989;25(3):335–48.
Åström KJ, Hägglund T. The future of PID control. Control Eng Pract. 2001;9(11):1163–75.
Lee SL, O’Connor TF, Yang X, Cruz CN, Chatterjee S, Madurawe RD, et al. Modernizing pharmaceutical manufacturing: from batch to continuous production. J Pharm Innov. 2015;10(3):191–9.
Mascia S, Heider PL, Zhang H, Lakerveld R, Benyahia B, Barton PI, et al. End-to-end continuous manufacturing of pharmaceuticals: integrated synthesis, purification, and final dosage formation. Angewandte Chemie (International ed in English). 2013;52(47):12359–63.
Içten E, Giridhar A, Taylor LS, Nagy ZK, Reklaitis GV. Dropwise additive manufacturing of pharmaceutical products for melt-based dosage forms. J Pharm Sci. 2015;104(5):1641–9.
Baronsky-Probst J, Möltgen C-V, Kessler W, Kessler R. Process design and control of a twin screw hot melt extrusion for continuous pharmaceutical tamper-resistant tablet production. Eur J Pharm Sci. 2016;87:14–21.
Hsu S-H, Reklaitis GV, Venkatasubramania V. Modeling and control of roller compaction for pharmaceutical manufacturing. J Pharm Innov. 2010;5(1–2):24–36.
Hsu S-H, Reklaitis GV, Venkatasubramanian V. Modeling and control of roller compaction for pharmaceutical manufacturing. Part I: process dynamics and control framework. J Pharm Innov. 2010;5(1–2):14–23.
Myerson AS, Krumme M, Nasr M, Thomas H, Braatz RD. Control systems engineering in continuous pharmaceutical manufacturing. May 20–21, 2014 continuous manufacturing symposium. J Pharm Sci. 2015;104(3):832–9.
Singh R, Boukouvala F, Jayjock E, Ramachandran R, Ierapetritou M, Muzzio F. Flexible multipurpose continuous processing of pharmaceutical tablet manufacturing process. GMP News, European Compliance Academic (ECE) 2012.
Singh R, Muzzio FJ, Ierapetritou M, Ramachandran R. A combined feed-forward/feed-back control system for a QbD-based continuous tablet manufacturing process. Processes. 2015;3(2):339–56.
Jolliffe HG, Gerogiorgis DI. Process modelling and simulation for continuous pharmaceutical manufacturing of ibuprofen. Chem Eng Res Des. 2015;97:175–91.
Shi Z, McGhehey KC, Leavesley IM, Manley LF. On-line monitoring of blend uniformity in continuous drug product manufacturing process—the impact of powder flow rate and the choice of spectrometer: dispersive vs. FT. J Pharm Biomed Anal. 2016;118:259–66.
Razavi SM, Callegari G, Drazer G, Cuitiño AM. Toward predicting tensile strength of pharmaceutical tablets by ultrasound measurement in continuous manufacturing. Int J Pharm. 2016;507(1):83–9.
Singh R, Román-Ospino AD, Romañach RJ, Ierapetritou M, Ramachandran R. Real time monitoring of powder blend bulk density for coupled feed-forward/feed-back control of a continuous direct compaction tablet manufacturing process. Int J Pharm. 2015;495(1):612–25.
Godoy J, González A, Normey-Rico J. Constrained latent variable model predictive control for trajectory tracking and economic optimization in batch processes. J Process Control. 2016;45:1–11.
Sanders CF, Hounslow MJ, Doyle FJ. Identification of models for control of wet granulation. Powder Technol. 2009;188(3):255–63.
Mesbah A, Nagy ZK, Huesman AE, Kramer HJ, Van den Hof PM. Nonlinear model-based control of a semi-industrial batch crystallizer using a population balance modeling framework. IEEE Trans Control Syst Technol. 2012;20(5):1188–201.
Kwon JSI, Nayhouse M, Christofides PD, Orkoulas G. Modeling and control of protein crystal shape and size in batch crystallization. AICHE J. 2013;59(7):2317–27.
Dejardin N, Alamir M, Corriou J. Model predictive control of a simulated moving bed. IFAC Proceedings Volumes. 2005;38(1):91–6.
Daraoui N, Dufour P, Hammouri H, Hottot A. Model predictive control during the primary drying stage of lyophilisation. Control Eng Pract. 2010;18(5):483–94.
Dufour P. Control engineering in drying technology: review and trends. Dry Technol. 2006;24(7):889–904.
Sen M, Singh R, Ramachandran R. A hybrid MPC-PID control system design for the continuous purification and processing of active pharmaceutical ingredients. Processes. 2014;2(2):392–418.
Rehrl J, Kruisz J, Sacher S, Khinast J, Horn M. Optimized continuous pharmaceutical manufacturing via model-predictive control. Int J Pharm. 2016;510(1):100–15.
Singh R, Ierapetritou M, Ramachandran R. System-wide hybrid MPC–PID control of a continuous pharmaceutical tablet manufacturing process via direct compaction. Eur J Pharm Biopharm. 2013;85(3, Part B):1164–82.
Hattou S, Lann M-VL, Preuss K, Roussel B, Cabassund M. Implementation of model predictive controller in a pharmaceutical development plant. In: Pistikopoulos EN, Georgiadis MC, Kokossis AC, editors. 21st European symposium on computer aided process engineering. Oxford, UK: Elsevier; 2011. p. 502–6.
Laurí D, Lennox B, Camacho J. Model predictive control for batch processes: ensuring validity of predictions. J Process Control. 2014;24(1):239–49.
Jingyi L, Zhixing C, Furong G. A stable two-time dimensional (2D) model predictive control with zero terminal state constraints for constrained batch processes. IFAC-PapersOnLine. 2015;48(8):514–9.
Singh R, Sahay A, Muzzio F, Ierapetritou M, Ramachandran R. A systematic framework for onsite design and implementation of a control system in a continuous tablet manufacturing process. Comput Chem Eng. 2014;66:186–200.
Román-Ospino AD, Singh R, Ierapetritou M, Ramachandran R, Méndez R, Ortega-Zuñiga C, et al. Near infrared spectroscopic calibration models for real time monitoring of powder density. Int J Pharm. 2016;512(1):61–74.
Kalman RE. A new approach to linear filtering and prediction problems. J Basic Eng. 1960;82(1):35–45.
Acknowledgements
This work is supported by the National Science Foundation Engineering Research Center on Structured Organic Particulate Systems, through grant NSF-ECC 0540855, Rutgers Research Council, through grant 202342 RC-17 to Singh R, and by the US Food and Drug Administration (FDA), through grant 11695471.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Haas, N.T., Ierapetritou, M. & Singh, R. Advanced Model Predictive Feedforward/Feedback Control of a Tablet Press. J Pharm Innov 12, 110–123 (2017). https://doi.org/10.1007/s12247-017-9276-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-017-9276-y