Abstract
To uncover the nature of various kinds of stolons of Utricularia subgenus Polypompholyx (Lentibulariaceae) we studied branching of stolons by scanning electron microscopy, statistically investigated correlations of stolon types and other traits across 56 species, and evaluated seedling development and process morphological aspects. Some results were compared to the sister genera Pinguicula and Genlisea. A key to nine stolon types in Polypompholyx is provided. Predominant stolon types were rhizoids, runner stolons with rhizoids on nodes, and runner stolons without rhizoids on nodes but with bladders on internodes. Stolon types were taxonomically relevant and correlated to the distribution/climate. They obviously diverged with speciation events in Australia. Examined seedlings of Genlisea and Polypompholyx showed similar developmental patterns. Stolons were homologous to traps and leaves. Selected subterranean organs contained specific but similar process combinations of roots, shoots and/or leaves. We assume the Genlisea-Utricularia ancestor trap included processes of a Pinguicula root and leaf.
Similar content being viewed by others
Introduction
The Lentibulariaceae (Lamiales) with its genera Pinguicula L., Genlisea A.St.-Hil. and Utricularia L. is the largest family of carnivorous plants. The monophyletic Pinguicula is predominantly represented in the Northern Hemisphere, in Central America, the Caribbean, and along the eastern regions of South America, growing generally in a variety of moist to wet habitats and in cavities of calcareous rocky slopes (e.g. Legendre, 2000; Fleischmann & Roccia, 2018). The genus possesses aerial leaves capable of trapping and digesting small animals by stalked and sessile trichomes, respectively. The “conventional” nutrient uptake is via roots, although, depending on the species, Pinguicula roots may be morpho-anatomically reduced (e.g. without root cap and/or branching) and ephemeral (Legendre, 2000; Adlassnig et al., 2005; Reut et al., 2021). Genlisea and Utricularia show several morphological similarities and phylogenetically form a sister clade of Pinguicula (Jobson et al., 2003; Müller & Borsch, 2005). Genlisea occurs in tropical regions of Central and South America, Cuba, Africa, and Madagascar, and is confined to nutrient poor, usually wet to often submerged or inundated habitats (Fleischmann, 2012, 2018), where it catches tiny invertebrates living in wet soil or water (Płachno et al., 2005; Fleischmann, 2012). With around 240 species, Utricularia (bladderworts) is the most diverse genus of the family. It is almost globally distributed in a large variety of nutrient deficient wetlands such as bogs, wet and inundated depressions, riverbanks, lakeshores, wet heaths, sandy to peaty soils, and rocky surfaces, but a minority of obligate hydrophytes occupies also inland waters (Taylor, 1989; Guisande et al., 2007; Jobson et al., 2018b; Adamec, 2020; Reut et al., 2021). The genus is subdivided into Polypompholyx (Lehm.) P. Taylor, Bivalvaria Kurz and Utricularia P.Taylor, with Polypompholyx being monophyletic (Müller & Borsch, 2005; Jobson et al., 2018b; Silva et al., 2018). As we currently know, subgenus Polypompholyx encompasses sections Polypompholyx (Lehm.) P. Taylor with two, Tridentaria P. Taylor with one, Pleiochasia Kamiénski with 29, and Lasiocaules R.W.Jobson & Baleeiro with 24 recognized species (Jobson et al., 2017, 2018a; Jobson & Baleeiro, 2020; Jobson & Cherry, 2020; Baleeiro & Jobson, 2022).
In Pinguicula and Genlisea, the most common life cycle strategy is perennial (Fleischmann, 2018; Fleischmann & Roccia, 2018), and although almost 50% of Utricularia species are annual, the perennial life cycle appears to be their ancestral state (Jobson et al., 2003). There are species in Genlisea and Utricularia exhibiting both life cycles (Taylor, 1989; Fleischmann et al., 2010), which may result from different habitat or climatic conditions. Shifts from one life cycle to another may occur in one species as response to environmental changes and not necessarily as evolutionary trend (Wang et al., 2016). In Utricularia subg. Polypompholyx, except for U. dichotoma subsp. novae-zelandiae (Hook.f) R.W.Jobson, which is distributed in New Zealand and New Caledonia, species occur exclusively in Australia where they occupy moist to submerged habitats (Jobson & Baleeiro, 2020). Jobson et al. (2017) concluded that the predecessor of Polypompholyx was annual, and that all species of sections Polypompholyx and Tridentaria, and merely all taxa of section Lasiocaules have an annual lifecycle. The authors showed that the life history in Polypompholyx is correlated with biogeography and seasonality, and that annual species occur predominantly in seasonally drier and monsoonal regions, while perennial species are more abundant in permanently wet habitats or temperate regions of Australia (Jobson et al., 2017).
The Vegetative Body of Genlisea and Utricularia
In Genlisea and Utricularia, the trapping function has been evolutionary transferred from aerial leaves of a Pinguicula-[Genlisea-Utricularia] ancestor into subterranean, ascidiate leaf-like organs. It seems that while adding complexity to the trap morphology, the sister genera Genlisea and Utricularia abandoned or reduced some other structures known in Pinguicula (e.g. of the root) that were of less importance or were replaced by the trap function (e.g. uptake of nutrients) for a successful propagation (Rutishauser, 2016, 2020; Reut & Płachno, 2020; Reut et al., 2021). The traps of Genlisea and Utricularia deploy the functionality in moist to submerged habitats, with Utricularia being physiologically the most dependent on free water (e.g. Juniper et al., 1989; Guisande et al., 2007; Płachno et al., 2014). In Utricularia, the traps (bladders) show a taxonomically relevant variety of the entrance architecture and of external appendages (e.g. Taylor, 1989; Poppinga et al., 2016; Westermeier et al., 2017; Jobson et al., 2018b; Płachno et al., 2019). However, bladder structures may relate to the substrate in terms of mechanical protection and prey capturing mechanisms (in soil versus water), and are, therefore, also considered to be correlated to life forms (Reut & Jobson, 2010; Westermeier et al., 2017).
Apart from the homologous traps, Genlisea and Utricularia have several other features in common that substantiate a close relationship between the two genera (Fleischmann, 2018; Jobson et al., 2018b). Genlisea has a rudimentary vegetative body of a short stem with rosulate aerial leaves and only traps growing into the substrate. The simplest vegetative organization in Utricularia is found in several rosulate species of subgenus Polypompholyx, which bear two types of geotropically positive organs: traps and unbranched rhizoids (anchor stolons). Initial morphological and anatomical simplifications from Pinguicula to the Genlisea-Utricularia ancestor may have been fostered in hydric environments with variable water conditions, and they culminated in a miniaturization of the genome, which is unrivalled in the plant kingdom (cf. Hidalgo et al., 2015; Greilhuber et al., 2006; Veleba et al., 2014; Reut & Płachno, 2020). However, Utricularia further evolved increasingly complex vegetative organs (e.g. runner stolons, water shoots, air shoots, and floaters), the more it adapted to submergence and to a life in the water column (cf. Taylor, 1989; Jobson et al., 2018b; Reut et al., 2021).
In Polypompholyx, the predominant and plesiomorphic habit (life form) is amphibious (“terrestrial”), but we presently also know eight emergent hydrophytes (“affixed subaquatics”), five submerged hydrophytes (“affixed aquatics”), one lithophytic (U. wannanii R.W.Jobson & Baleeiro), and one free-floating (freely suspended) aquatic species (U. tubulata F.Muell.) (Taylor, 1989; Jobson et al., 2017, 2018b; Reut et al., 2021). Reut et al. (2021) investigated morpho-anatomical adaptations of Utricularia (including 7 taxa of subgenus Polypompholyx) to submergence and a life in the water column and found that leaves tend to be narrower in submerged hydrophytes and dissected in free-floating hydrophytes, confirming similar observations on heterophyllous amphibious plants (e.g. Nakayama et al., 2017) and obligate hydrophytes (e.g. Sculthorpe, 1967; Colmer et al., 2011). An increased submergence may, furthermore, induce the elongation of stolons and internodes (Wetzel, 1988; Voesenek et al., 2006).
Developmental and Dynamic Morphology
Goebel (1891) insinuated that the understanding of the vegetative morphology is elucidated by grasping the embryo and seedling development. The family Lentibulariaceae shows an evolutionary trend in embryo simplification by the loss of a radicle (in Genlisea and Utricularia) and the reduction of cotyledons (e.g. Merl, 1915; Degtjareva et al., 2004, 2006; Płachno & Świątek, 2010; Fleischmann, 2012). In Pinguicula, one or two cotyledons are developed (Degtjareva et al., 2004), and in Genlisea the cotyledons are thought to remain in the seed testa (Merl, 1915; Fleischmann, 2012) or grow as small structures (Fleischmann, 2012). Although Utricularia is a species rich genus, only about 10% of all taxa were studied regarding germination patterns (e.g. Warming, 1874; Kamieński, 1876; Goebel, 1891; Lang, 1901; Merl, 1915; Lloyd, 1937; Kondo et al., 1978; Brugger & Rutishauser, 1989; Płachno & Świątek, 2010; Studnička, 2011). In Utricularia, the embryo is the most reduced, except for the aquatic epiphytes U. nelumbifolia Gardner, U. cornigera Studnička, and U. humboldtii Schomb., which display seedlings with 6–15 chlorophyllous, flattened or forked primary organs (Merl, 1915: “primary leaves”; Lloyd, 1942: “cotyledonoids”; Płachno & Świątek, 2010; Studnička, 2011). However, these primary organs do not appear to be cotyledons (Compton, 1909; Płachno & Świątek, 2010), and Kondo et al. (1978) considered Utricularia to be acotyledonous.
The floral bauplan of Utricularia is conservative and conforms to the Lamiales and other angiosperms, but the vegetative morphology of the genus follows structural rules differing from typical flowering plants (Rutishauser & Sattler, 1989; Rutishauser & Isler, 2001; Rutishauser, 2016, 2020). Bladderworts are regarded as root-less (Taylor, 1989; Adlassnig et al., 2005). Some authors even considered them as being leaf-less, having leaf-like structures representing phylloclades (cf. Compton, 1909; Troll & Dietz, 1954; Płachno & Świątek, 2010) or fuzzy organ identity, combining shoot and leaf characters (Rutishauser, 2016, 2020). There are fundamental theories behind these views.
While the Goethean idealistic plant morphology relies on the concept that plants consist of parts, which are phylogenetically determined and derive from a basic organ class (i.e. either ‘root’, ‘stem’, or ‘leaf’), the realistic (essentialistic) morphology emphasizes that ontogenetic processes influence the form, which may change by specialization of a basic organ class (Ganong, 1901, 1913). For instance, in the orchid Taeniophyllum Blume, photosynthesis is performed by flattened, chlorophyllous aerial roots, while foliar leaves were evolutionary abandoned. Ganong (1913: Fig. 25) used this organ of Taeniophyllum as an example of morphological origin (traceable back to a root) interrelated with ecological meaning (collecting light by a leaf). Although these classical morphological concepts are still in many minds, none of these theories acknowledges that organs can have multiple identities or partial homologies by the transfer of functions and genetically determined traits (cf. Baum 2019). Continuum and process plant morphology are modern approaches to understand intermixing of classical morphological categories of leaves, shoots and roots (cf. Rutishauser, 2016, 2020), which are determined by developmental processes influenced by the expression and repression of corresponding genes (cf. Chormanski & Richards, 2012; Ibarra-Laclette et al., 2013; Carretero-Paulet et al., 2015a, 2015b).
Addressing the phenotypic plasticity of U. dichotoma Labill. sensu lato, Reut & Płachno (2020) examined developmental patterns and the anatomy of the vegetative body from 25 sources from Australia and New Zealand. The authors demonstrated that there is a common pattern of initial stolon node formation, and a high degree of interchangeability of organ types in later stolon node development. Reut & Płachno (2020) visualized the morphospace of vegetative organs of U. dichotoma by means of the Principal Component Analysis (PCA), methodologically largely following Jeune & Sattler (1992). The results showed that the structure and function of each organ are influenced by combinations of developmental processes for root, shoot and leaf, and that strict boundaries between organ categories are blurred (cf. Jeune & Sattler, 1992; Sattler & Jeune, 1992; Jeune et al., 2006; Kirchoff et al., 2008).
Variety of Stolons in Polypompholyx
The rosette plants of subgenus Polypompholyx sect. Polypompholyx and sect. Tridentaria were suspected to be the most “primitive” representatives of Utricularia, as they share more morphological similarities with Genlisea than other species of the genus (cf. Merl, 1915; Lloyd, 1942; Fleischmann et al., 2010; Jobson et al., 2018b). However, a phylogenetic affinity of Polypompholyx with the Genlisea-Utricularia ancestor has not been substantiated to date.
In contrast to Pinguicula and Genlisea, most Utricularia species (and subgenus Polypompholyx) are stoloniferous (Taylor, 1989; Jobson et al., 2018b). Merl (1915) suggested that stoloniferous species of section Pleiochasia (sensu Jobson et al., 2017) represent a transitional stage towards more advanced terrestrial bladderworts. However, while the ancestor of the genus Utricularia may have already shown stolonifery, a closer morphological affinity of rosulate Polypompholyx species to Genlisea may likewise be a result of retrogressive evolution towards a rosulate Genlisea-Utricularia ancestor (cf. Jobson et al., 2018b). Irrespective of the origin of stolonifery, only subgenus Polypompholyx offers the opportunity to study simple (non-stoloniferous) and complex growth forms in one taxonomic group within Utricularia.
The variability and variety of stolons in subgenus Polypompholyx is remarkable. There are unbranched rhizoids and stolons bearing bladders along their axis (‘simple stolons’), whereas the latter type may develop from the former when the water table is increased (Reut & Płachno, 2020). Other stolons show branching patterns with a seemingly high phenotypic plasticity and with unknown genotypic determination (cf. Taylor, 1989). Stolons of sect. Lasiocaules seem to differ from stolons of sect. Pleiochasia by having bladders on internodes (Jobson et al., 2017). Apart from U. dichotoma sensu lato (Reut & Płachno, 2020), no systematic review has been undertaken within Polypompholyx to reveal branching patterns and organogenesis on stolons of stoloniferous species.
Focussing on the diversity of stolons in subgenus Polypompholyx, we aim to better understand branching patterns, correlations to other characters or external factors, taxonomic and evolutionary aspects, the early development on seedlings, and process morphology. Correlations between stolon type and phylogenetic group, distribution/climate, leaf form and apex, trap type, plant and stolon size, life form, and life cycle across all species are evaluated, based upon information from the literature, and statistically assessed by PCA. Early developmental stages of seedlings of U. westonii P.Taylor (sect. Tridentaria) and G. pygmaea A.St.-Hil. are studied by scanning electron microscopy (SEM) to reveal possible patterns and homologies of stolons to other organs. The dynamic morphology of stolons, other vegetative organs of Polypompholyx, the trap of Genlisea repens Benj., and the root of Pinguicula gigantea Luhrs is evaluated by applying a PCA with developmental processes (cf. Reut & Płachno, 2020).
We acknowledge that naming organs in Polypompholyx, that have an unclear or mixed identity, poses challenges, but for reason of consistency and in agreement with e.g. Taylor (1989) and Lloyd (1942), we use terms that have been established in the past. In this paper, we distinguish runner stolons (syn. runners) from other types of stolons, whereby runner stolons produce nodes with rosettes of various organs and (generally) inflorescences.
Materials and Methods
Plant Material and Preparation
Morphological studies were done by SEM on 12 taxa to represent Pinguicula, Genlisea, and three out of four sections of Utricularia subg. Polypompholyx (see Table 1 for taxonomic information, sources, and habits of the material). Utricularia westonii was selected to investigate early developmental stages and patterns of seedlings, which were compared to seedlings and rhizomes of Genlisea pygmaea used in an earlier study (Reut, 1993b).
The material of Pinguicula and Utricularia was carefully rinsed, cleaned from debris, fixed as in Reut et al. (2021), and pre-examined under a stereo microscope. A part of the material was selected for further examination, dehydrated, and critically-point dried using liquid CO2. Subsequently, it was sputter-coated with gold and examined with a Hitachi S-4700 scanning electron microscope at the Institute of Geological Sciences, Jagiellonian University in Kraków, Poland. Genlisea pygmaea material was fixed in 70% ethanol with 1% glycerine, proceeded as above, and studied with a Hitachi S-4000 scanning electron microscope at the Institute of Systematic Botany, University of Zürich, Switzerland in 1992.
In addition, plants of Trapa natans L. were collected from a pond of the Botanic Garden of Jagiellonian University in Kraków, Poland, in September 2021 to study the anatomic origin of branches in submerged photosynthetic roots. Pictures of the plants were taken with a Galaxy S105G phone. Samples for SEM examinations were treated as described above. Cross sections were prepared and investigated as published in Gomes Rodrigues et al. (2017). Handmade sections were treated with alum carmine and iodine green for staining of lignin and cellulose. A Nikon Eclipse E400 light microscope with UV-2A, DAPI filter was used for observations and photo documentation.
Data Collection
Multivariate analyses were performed to assess a) correlations of biometric traits (clade/phylogeny, region/distribution, life forms/habits, life cycle, plant size, leaf form and apex, trap morphology, and stolon length and type) across all 56 known species of Utricularia subg. Polypompholyx, and b) correlations of developmental processes of the root of Pinguicula gigantea, the trap (rhizophyll) of Genlisea, organs of Utricularia subg. Polypompholyx, and organs of twelve other angiosperms. Supplementary Information 1 (online resource) contains data matrices of samples (species/organs) and variables (biometric traits/developmental processes) with resulting values used in the multivariate analyses. We ran PCAs including biplots with BioVinci (version 3.0.9, BioTuring Inc) and XLSTAT statistical and data analysis solution (2022.2.1, Addinsoft, Boston, USA, https://www.xlstat.com). Linear regressions were calculated in Microsoft Excel. The PCA with confidence ellipses was done with Clustvis (Metsalu & Vilo, 2015).
Data used for the determination of stolon types and for the multivariate analysis of various biometric traits in subgenus Polypompholyx were gathered from morphological examinations using scanning electron microscopy on seven stoloniferous taxa, earlier works by Merl (1915) and Lloyd (1942), the monograph of genus Utricularia (Taylor, 1989), and subsequent species/section descriptions (Gassin, 1993; Lowrie, 1998, 2002; Wakabayashi, 2010; Jobson, 2012, 2013; Jobson et al., 2017, 2018a; Jobson and Baleeiro, 2015, 2020; Jobson & Cherry, 2020, Baleeiro & Jobson, 2022). Values of several traits were pre-evaluated to select the strongest contributing traits for the PCA. For this reason, we preferred the seven clades of Polypompholyx (cf. Jobson et al., 2017) to the four sections of Polypompholyx as variable ‘clade’, and characters of trap wings to combinations with lateral appendages as variable ‘trap type’. As rhizoids occurred in all species of subgenus Polypompholyx, except for U. tubulata F.Muell., we used only “main” stolons (horizontal or floating) with highest branching complexity as character ‘stolon type’. In the literature, more information was available on stolon length than on internode length. Since we found that stolon and internode lengths generally directly correlate, we decided to use ‘stolon length’ as a trait in the analysis. Where no data on stolon length was available (U. dichotoma, U. speciosa R.Br., U. oppositiflora R.Br., U. holtzei F.Muell., U. singeriana F.Muell., and U. tridactyla P.Taylor), the stolon length was approximated based on descriptions or drawings of internodes as provided in Taylor (1989) and Jobson & Baleeiro (2020). Table 2 shows the finally defined biometric traits (variables) and their values used for the multivariate analysis.
Table 3 lists the plant species and organs (with notes on general characteristics and the source of information) used for the multivariate analysis of organ classes in angiosperms. Table 4 shows the selected developmental processes and their corresponding values in organs, largely following Reut & Płachno (2020) with slight modifications on ‘expansion’ (apical/subapical/transverse expansion has been separated from longitudinal/lateral/marginal expansion, providing more variability) and ‘vascular tissue distribution’ (the process for absent vascular elements or not identifiable distribution of vascular tissue was omitted, as it was not applicable for the scope of the current study). The developmental process ‘branching complexity’ was added to this analysis, taking account of non-branching organs and branching with single organs, multiple branching on nodes, or with mixed branching patterns. This allowed, for instance, to differentiate between stolon types in Utricularia subg. Polypompholyx.
Results
Typology and Morphology of Stolons
Based upon the literature review and the current morphological investigations of subgenus Polypompholyx, we identified nine types of stolons, for which we propose the following key.
-
1.
Stolon without adaxial rosettes bearing vegetative organs and inflorescences ………………………………3
-
2.
Stolon with adaxial rosettes of various organs (runner stolon) ……………………………………………...8
-
3.
Stolon without daughter structures ……………………………… rhizoid (syn. ‘anchor stolon’)
-
4.
Stolon with a row of single bladders ……………..…… bladder-bearing rhizoid (syn. ‘simple stolon’)
-
5.
Stolon with a row of single or paired organs other than just bladders …………………. type ‘lasiocaulis’
-
6.
Stolon with bladders and leaves alternating in whorls on stolon nodes ………………….. type ‘tubulata’
-
7.
Runner stolon without organs on internodes …………………………………………………………………..9
-
8.
Runner stolon with organs on internodes …………………………………..…………………………………12
-
9.
Runner stolon without organs on internodes, without rhizoids ……………………………… type ‘holtzei’
-
10.
Runner with a row of tufts of bladders (and occasionally with rosettes) ………..…..….. type ‘volubilis’
-
11.
Runner stolon without organs on internodes, with rhizoids …………………………………. type ‘dichotoma’
-
12.
Runner stolon with organs on internodes, without rhizoids ………………………………………. type ‘uniflora’
-
13.
Runner stolon with organs on internodes, with rhizoids …………………………………………… type ‘magna’
Six of these stolon types were found in the material of U. volubilis R.Br., U. paulineae A. Lowrie, U. dichotoma, U. oppositiflora, and U. beaugleholei R.J. Gassin of section Pleiochasia, and U. uniflora R.Br. and U. magna R.W.Jobson & M.D.Barrett of section Lasiocaules), examined by SEM and described in detail as follows.
Utricularia volubilis
Plants of U. volubilis exhibited three types of stolons (Fig. 1a–c), which all grew by apical elongation: a) very few c. 0.4 mm thick branched stolons of type ‘dichotoma’ with rosettes bearing leaves, bladders, rhizoids, and daughter stolons, b) 0.3–0.4 mm thick stolons of type ‘volubilis’ bearing whorls of 2–6 bladders, and c) few c. 0.3 mm thick rhizoids.
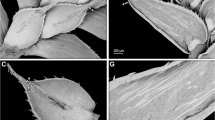
Organ development on stolons in Utricularia section Pleiochasia. a Apical part of a U. volubilis stolon of type ‘volubilis’, displaying a subapical hump (h”) and, more distal from the tip, another hump (h’) with three primordia. b Hump with five bladders (b1-5) on the same type of stolon. c Runner stolon of type ‘dichotoma’ of U. volubilis with a rosette arising inverse axillary at the base of a subtending leaf (L1). The rosette shows two leaves (L) flanked by indeterminate organs (maybe runner stolons, R?), another somewhat flatter organ (maybe a juvenile leaf, L?), and groups of primordia (asterisks) at the base of each leaf. d Stolon of U. paulineae with a rosette of leaves, a bladder (b), an indeterminate primordial organ (maybe developing to a bladder, bp?), and a primordial leaf (Lp) with a group of three primordia (asterisk) at its base and a detached organ (x) on the opposite side of Lp. e Stolon rosette of U. paulineae showing three leaves, a bladder, a rhizoid (a), an indeterminate juvenile organ in the centre of the rosette (asterisk), and two other indeterminate juvenile organs (arrowheads). f Apical part of a runner stolon of U. dichotoma subsp. monanthos with a strongly coiled tip and three primordia (asterisk) close to it. Distal from the tip, a rosette is visible, consisting of an inverse-axillant subtending leaf, two flanking bladders (b2 and b3), and three primordia (4–6). g Juvenile bladders and primordia (asterisk) in the curved apical part of ‘simple stolons’ (s) of U. oppositiflora. Arrows point in the direction of the tip of the main stolon. Primordia and organs are numbered according to the order of development in rosettes. Scale bars = 0.5 mm in a, d, g; 1 mm in b, c, e; 0.3 mm in f
In intervals along some unbranched stolons of type ‘volubilis’, 2–6 bladders grew almost simultaneously as whorl from a hump (a compressed side shoot of the stolon) (Fig. 1a, b). The axis of the whorl was approximately in a 120–135° angle to the growing direction of the mother stolon axis. The succession of organs on the whorl seemed to be irregular but was often initiated by two distichously positioned bladder primordia and a third primordium, which was slightly more proximal to the stolon tip. Bladder petioles grew up to 2–4 mm, before the bladders reached a mature and functional stage. The distal part (towards the tip) of some of these stolons appeared somewhat bent, while the stolon tip was straight.
On the adaxial side along other branched stolons of type ‘dichotoma’ (Fig. 1c), meristematic zones (‘nodes’, ‘humps’, ‘compressed side shoots’) were formed. They developed leaves, and in their proximal axils, various further organs arose in seemingly irregular but probably spiral or centripetal order. Stolon tips were straight.
Utricularia paulineae
The material of U. paulineae revealed two stolon types: a) 0.2–0.3 mm thick main stolons of type ‘dichotoma’ with rosettes bearing leaves, bladders, and rhizoids, and b) 0.1–0.2 mm thick rhizoids.
Main stolons developed short side shoots with organs arising in irregular but probably spiral-centripetal order (Fig. 1d, e). An axillant leaf on the rosette could not be identified. Due to damaged and sparse material, we were not able to see early stages of rosettes closer to the stolon tips.
Utricularia dichotoma, U. oppositiflora, and U. beaugleholei
Three stolon types were found in the three taxa: a) 0.2–0.4 mm (up to 0.6 mm in U. oppositiflora) thick branched stolons of type ‘dichotoma’ (Fig. 1f), carrying leaves, bladders, ‘simple stolons’, and daughter runners, b) 0.1–0.2 mm (up to 0.3 mm in U. oppositiflora) thick ‘simple stolons’ bearing bladders (Fig. 1g), and c) 0.1–0.2 mm thick rhizoids, which were found at the base of the peduncle.
Rosettes on branched stolons were initiated by the formation of a leaf and two almost laterally flanking bladders on the adaxial side of the stolon. In the proximal axil of the first leaf, a bud with two distichously arranged ‘simple stolons’ and, somewhat closer to the leaf base, up to three primordia developed (Fig. 1f). Tips of branched stolons were coiled. ‘Simple stolons’ of U. oppositiflora were curved, differing from the generally straight tips of ‘simple stolons’ of U. dichotoma and U. beaugleholei.
In their juvenile stage, ‘simple stolons’ appeared as (often twisted) rhizoids, but during elongation they grew geotropically positively into the substrate and formed up to four bladders in monostichous organotaxis.
Utricularia uniflora
The plants of U. uniflora exhibited two stolon types: a) 0.1–0.2 mm thick branched stolons of type ‘uniflora’ (Fig. 2a–c) with leaves, bladders and daughter stolons, and b) 0.1–0.15 mm ‘simple stolons’ bearing bladders.
Morphology of runner stolons (R) in Utricularia section Lasiocaules. a Bladders growing monostichously along a stolon (type’uniflora’) of U. uniflora. Juvenile bladders (b) are found towards the first stolon rosette (r). Primordial bladders (bp) are present proximal to the stolon tip, where they are initially formed (arrowhead). b Runner stolon of U. uniflora showing a rosette and a mature bladder (bi) on an internode. The rosette shows a subtending leaf (L1) in inverse axillant position, a bladder (br), and three indeterminate organs in the leaf axil. c Later developmental stage of a U. uniflora stolon node with four bladders and another runner stolon. The latter displays a mature bladder and insertions of apparently detached bladders (bx). One organ (x, probably the subtending leaf) got detached during preparation. d Early developmental stages of a U. magna runner stolon (type’magna’), exhibiting a rosette with an inverse axillant subtending leaf (L1), a detached organ (2), and two primordia (3, 4) at the base of the leaf, an internode with one solitary bladder, and a primordium (asterisk) at the stolon tip (presumably initiating a stolon node). e Runner stolon of U. magna showing the succession of two rosettes displaying a subtending leaf, a rhizoid (a2), a second leaf (L3), and primordia (asterisk) in the axil of L1. A single insertion (most likely a detached bladder, bx) is seen on the internode. f Advanced stolon rosette of U. magna with numerous leaves, rhizoids (a), and 1–2 juvenile inflorescences and 1–2 bladders in the centre (asterisk) of the rosette. Arrows point in the direction of the tip of the main stolon. Primordia and organs are numbered according to the order of development in rosettes. Scale bars = 1 mm in a-c, e, f; 0.2 mm in d
Branched stolons had single bladders and occasionally single leaves along internodes on the adaxial side, and rosettes with random combinations of up to 7 leaves, up to 8 bladders, and occasionally 1–2 daughter stolons and/or ‘simple stolons’ on a short vertical stem. During longitudinal growth, ‘simple stolons’ potentially turned into branched stolons by building rosettes after approximately 2–10 organs (generally bladders) in distances of 2–5 mm had grown along the internode (Fig. 2a). Rosettes showed a subtending leaf in inverse axillant position (Fig. 2b). No rhizoids were observed on stolons. Stolon tips were straight.
Utricularia magna
The material of U. magna showed a) 0.15–0.2 mm thick stolons of type ‘magna’ with rosettes of leaves, bladders, rhizoids, and inflorescences, and with one bladder on the internode (Fig. 2d–f), and b) 0.05–0.1 mm thick rhizoids.
On the adaxial side of the runner stolon, a rosette was formed in inverse axillary position at the base of a subtending leaf. The rosettes initially developed a rhizoid, a second leaf, and three primordia between the two organs (Fig. 2d, e). A third leaf arose subsequently. The development of 1–2 inflorescences was initiated in the centre of the rosette after around 6 leaves were formed on the stolon node (Fig. 2f). Juvenile bladders were also visible at this later stage of rosette development. Runners showed straight tips.
Correlations of Stolon Types and Other Biometric Traits
In multivariate analyses, clustering of Polypompholyx species by phylogenetic clades was weakly supported (e.g. PCA with Silhouette Score c. 0.2), since the groups overlapped to some extent. The best supported grouping of species was achieved by computed auto-clustering with a Silhouette Score of c. 0.6. As a result, the PCA (Fig. 3) grouping was primarily driven by ‘clade’ and ‘stolon type’, and secondarily by ‘trap type’. If the variable ‘clade’ was omitted from the dataset, the graphical spreading and clustering of species in the PCA was only slightly altered (not shown here), since the directly correlated ‘stolon type’ had a similar effect. The PCA of the complete dataset led to following four solid clusters of species (Fig. 3).
Auto-clustering based PCA biplot of biometric traits and their correlations across 56 samples (species of Utricularia subgenus Polypompholyx) without scaling. Clust = cluster. Clust 0: U. albertiana (ALBE), U. antennifera (ANT), U. arnhemica (ARN), U. bidentata (BID), U. capilliflora (CAP), U. cheiranthos (CHE), U. dunlopii (DUNL), U. dunstaniae (DUNS), U. gaagudju (GAA), U. georgei (GEO), U. kamienskii (KAM), U. kenneallyi (KEN), U. kimberleyensis (KIM), U. lasiocaulis (LAS), U. leptorhyncha (LEP), U. magna (MAG), U. papilliscapa (PAP), U. quinquedentata (QUI), U. rhododactylos (RHO), U. tridactyla (TRID), U. uniflora (UNI), U. wannanii (WAN). Clust 1: U. albiflora (ALBI), U. baliboongarnang (BAL), U. byrneana (BYR), U. fistulosa (FIS), U. hamata (HAMA), U. hamiltonii (HAMI), U. holtzei (HOL), U. limmenensis (LIM), U. linearis (LIN), U. lowriei (LOW), U. singeriana (SIN), U. terrae-reginae (TER), U. triflora (TRIF), U. tubulata (TUB). Clust 2: U. benthamii (BEN), U. helix (HEL), U. inaequalis (INA), U. menziesii (MEN), U. multifida (MUL), U. petertaylorii (PET), U. tenella (TEN), U. violacea (VIO), U. volubilis (VOL), U. westonii (WES). Clust 3: U. ameliae (AME), U. barkeri (BAR), U. blackmanii (BLA), U. beaugleholei (BEA), U. dichotoma (DIC), U. fenshamii (FEN), U. grampiana (GRA), U. oppositiflora (OPP), U. paulineae (PAU), U. speciosa (SPE). Shapes of sample points represent species belonging to the same section/clade (cf. Jobson et al., 2017, 2018a; Jobson & Baleeiro, 2020; Jobson & Cherry, 2020; Baleeiro & Jobson, 2022): sect. Polypompholyx (P), sect. Tridentaria (T), sect. Pleiochasia (clades A-C), sect. Lasiocaules (clades D-F). Traits (see Table 2 for further information): clade/phylogeny (1), region/distribution (2), life form/habit (3), life cycle (4), plant size (5), leaf form (6), leaf apex (7), trap type (8), stolon length (9), stolon type (10). PC1 = 54.7%, PC2 = 16.4%, PC1-3 = 79.3%
Cluster 0 encompassed species mainly characterized by ‘stolon type’ (runners with organs on internodes, with or without rhizoids on nodes; stolons with rows of single or paired organs other than just bladders; or runners without organs on internodes and without rhizoid on nodes) and ‘clade’ (species of section Lasiocaules clades D, F and E, except for U. albertiana R.W.Jobson & Baleeiro and U. lowriei R.W. Jobson of clade E). Cluster 1 consisted of species of section Pleiochasia clade B (except for U. blackmanii R.W. Jobson), U. byrnea R.W. Jobson & Baleeiro and U. tubulata (clade C), and U. albertiana and U. lowriei (clade E) of section Lasiocaules, largely distinguishable by ‘trap form’ (reduced to generally absent or ± entire wings). Cluster 2 contained species primarily characterized by ‘region’ (SW Australia), ‘stolon type’ (rosulate plants, only with rhizoids from a short stem), and ‘clade’ (sections Polypompholyx, Tridentaria and Pleiochasia clade A, except for U. paulineae). Cluster 3 was composed of species of section Pleiochasia clade C, U. paulineae (clade A), and U. blackmanii (clade B), mainly defined by ‘life cycle’ (perennial, or annual or perennial) and ‘stolon type’ (runners without organs on internodes, and with rhizoids on nodes).
The prevalent stolon types were type ‘dichotoma’ with 19 species and type ‘uniflora’ with 16 species (Supplementary Information 1). Other stolon types were less frequent in the subgenus: nine rosulate taxa without horizontal (main/runner) stolons (i.e. with only rhizoids), followed by type ‘holtzei’ with six species, type ‘lasiocaulis’ with four species, and three types (‘volubilis’, ‘tubulata’, and ‘magna’) with one species each. Stolon type ‘dichotoma’ was predominant in clade C but occurred also in clade B of sect. Pleiochasia and in only two species of clade E of sect. Lasiocaules. It was restricted to clusters 1 and 3. Stolon type ‘uniflora’ prevailed in clades E and F of sect. Lasiocaules in cluster 0 but was also found in U. hamata R.W.Jobson & M.D.Barrett of clade B in sect. Pleiochasia in cluster 1. The absence of rhizoids on stolon nodes was predominant in sect. Lasiocaules but occurred also in clade B of sect. Pleiochasia. Similarly, single bladders on internodes were prevalent in sect. Lasiocaules, but in sect. Pleiochasia they were only present in U. hamata (cf. Jobson et al., 2018a) of clade B.
Table 5 provides the data of correlations of biometric traits calculated by linear regressions. We recognized four thematic correlation groups, within which the traits corresponded moderately to very strongly to each other: ‘correlation group phylogeny’ (‘clade’, ‘stolon type’, ‘leaf form’, ‘leaf apex’), ‘correlation group climate’ (‘region’, ‘clade’, ‘stolon type’), ‘correlation group habit’ (‘habit’, ‘leaf apex’, ‘leaf form’, ‘trap type’), and ‘correlation group reproduction’ (‘life cycle’, ‘stolon length’, ‘habit’, ‘plant size’).
Organogenesis in Seedlings
The seedling of U. westonii initially produced a primary leaf and an unbranched primary stolon (rhizoid). Between the primary leaf and the rhizoid but in slightly decentral position, at the base of the rhizoid, a bladder (trap) developed (Fig. 4a), followed by a second leaf at its base, approximately opposite from the primary stolon (Fig. 4b). Between the first bladder and the leaves, at the base of the bladder stalk, four more primordia were inserted (Fig. 4c, d).
Succession of primary leaf, primary rhizoid, primary bladder, and further organs on seedlings of U. westonii. a Early seedling stage, showing a primary leaf (L1) and a primary rhizoid (a2) with a primordium (3) at its base. b A slightly later stage of seedling growth with a primordium (4) between primary leaf and primordium 3. c Further developed seedling with primordia (asterisk) arising between primary bladder, primary leaf and second leaf (L4). d Close-up of c, showing the primordia (arrowheads) at the base of the primary bladder. Primordia and organs are numbered according to the order of development. Scale bars = 0.5 mm in a; 0.3 mm in b; 1 mm in c; 0.2 mm in d
In G. pygmaea, two organs penetrated the seed integument first. One of these organs developed into a primary foliage leaf, while the other organ (designated as “scale”) seemed to cease further growth. Early in development of the primary leaf, a primary trap appeared proximally on the petiole of the leaf. At a slightly later stage, the primary leaf flattened and grew geotropically negative, whilst the trap maintained a cylindrical shape and grew in the opposite direction. The further succession of organs (Fig. 5a–c) indicated that leaves and traps developed alternately and each at the base of the previous organ.
Developmental patterns on the seedling (a-c) and rhizome (d) of G. pygmaea, and root morphology of P. gigantea (e, f). a Seedling of G. pygmaea with primary leaf (L1), primary trap (T1), second leaf (T3), and second trap (T4). b Detailed view on the seedling with the “scale” (sc), the second trap growing at the base of the second leaf, and a primordium (5) developing at the base of the second trap. c A seedling showing the seemingly suppressed growth of organ 3 and the development of an organ (4) from the base of organ 3. A primordium (5) arises from organ 4. d Vegetative point of the rhizome tip, showing an irregular spiral but almost spiro-decussate organotaxis of the primordia 1–8. e Middle zone of the P. gigantea root with unicellular root hairs and papillae towards the root tip. f Glabrous apical zone of the root. Primordia and organs are numbered according to the order of development. Scale bars = 0.5 mm in a, f; 0.1 mm in b, c; 0.2 mm in d; 0.3 mm in e
Later in seedling development, the rhizome of G. pygmaea could be recognised as relative main axis. The lateral organs and the primordia on the vegetative point seemed to develop in irregular spiral but probably spiro-decussate order (Fig. 5d).
Developmental Processes of Organs
In contrast to the roots of Pinguicula (P. gigantea: Fig. 5e, f), none of the examined Utricularia species showed unicellular hairs on stolons or other submerged/subterranean organs. However, button-like trichomes were abundant on all submerged/subterranean organs, and often more frequent on the upper side of main stolons towards the tip. The examined feathery, photosynthetic root of Trapa natans (Fig. 6) did not exhibit root hairs either. Transverse sections through the submerged shoot (Fig. 6b) and the laterally arising roots (Fig. 6d) showed that that the “leaf-like” roots and their branches were borne endogenously.
Trapa natans. a Part of a submerged shoot with seemingly leaf-like (chlorophyllous) roots. Øb indicates the transverse plane through the shoot main axis as shown in b. b Cross section of the shoot displaying the central cylinder (cc) and a root branching endogenously (arrowhead). c Scanning electron microscopic image of a feathery (“pinnatisect”) root. Ød represents the transverse plane through the root as shown in d. d Cross section of a root main axis displaying a tetrarch stele and an endodermis with Casparian strips. Arrowheads point to zones where lateral roots were formed. b, d Autofluorescence of transverse sections under UV light. Scale bars = 1 cm in a; 0.5 mm in b; 1 mm in c; 0.1 mm in d
The plot of the PCA (Fig. 7) illustrates a morphospace of selected organs of Polypompholyx, Genlisea, Pinguicula and several other angiosperms defined by a combination (profile) of values of developmental processes. Organs with a similar profile are, therefore, located close to each other. Confidence ellipses (CI = 95%), circumscribing the “typical” organ classes roots, shoots and leaves, overlap between roots and shoots and between shoots and roots. Overlaps were smaller when unit variance scaling was applied in the pre-processing of the PCA (not shown here), but variances (principal components) as well as predictabilities, and therefore the interpretability, decreased. Organs appearing in the intersection of two ellipses generally combine developmental processes for both organ classes, whereas a sample has more in common with a specific organ class, the closer it is to the barycentre of the corresponding ellipse. Consequently, the feathery root of Trapa natans and the root of Taeniophyllum biocellatum J.J.Sm. had the closest affinity to a “typical” root, whilst the inflorescence of Trapa natans had the greatest correspondence with a “typical” shoot, and the leaf of Castanea sativa Mill. with a “typical” leaf.
PCA scatter plot of selected “typical” and “atypical” organs with confidence ellipses circumscribing “typical” roots, shoots, or leaves. The distribution of organs is based upon the correlations of 12 developmental processes as defined in Table 4. No scaling was applied to the PCA. Organs: leaf of Bauhinia purpurea (Bl), leaf of Castanea sativa (CSl), stolon of Centella asiatica (CAs), leaf of Chelidonium majus (CMl), shoot-like leaf of Chisocheton tenuis (CTl), trap of Genlisea repens (Gt), inflorescence of Lysimachia vulgaris (Ls), root of Nasturtium officinale (NOr), inflorescence of Nymphaea alba (Ns), leaf of Nymphaea alba (Nl), root of Pinguicula gigantea (Pr), stolon of Ranunculus repens (Rs), leaf of Ranunculus repens (Rl), root of Ranunculus repens (Rr), leaf of Sedum dasyphyllum (Sl), root of Taeniophyllum biocellatum (TBr), inflorescence of Trapa natans (Ts), leaf-like root of Trapa natans (Tlr), leaf of Utricularia dichotoma (UDl), ‘simple stolon’ of U. dichotoma (UDc), runner stolon of U. dichotoma (UDs), leaf of U. multifida (UMl), trap of U. multifida (UMt), rhizoid of U. multifida (UMa), stolon of U. uniflora (UUs). PC1 = 38.4%, PC2 = 20.7%, PC1-3 = 73.5%
The distribution of organs in the plot was mainly influenced by ‘growth period’ and the non-correlated ‘positioning’. Correlations of developmental processes were calculated by linear regression of pairs. The results are presented in Table 6, which demonstrates that significant correlations (p < 0.05) between developmental processes were e.g.: ‘positioning’, ‘vascular tissue distribution’ (direct); ‘branching origin’, ‘orientation’, ‘organotaxis’ (direct); ‘growth period’, ‘branching origin’ (indirect); ‘final symmetry’, ‘expansion’ (direct), ‘branching origin’ (indirect); ‘geotropism’, ‘vascular tissue distribution’ (indirect). Overall, the influence of ‘growth distribution’ and ‘branching complexity’ was relatively weak. ‘Developmental symmetry’ had almost no impact, as it had a very small variability (only Sedum dasyphyllum L. had an organ with changing symmetry).
Discussion
Variety of Stolons
The morphological investigation on various stolons occurring in subgenus Polypompholyx revealed several branching patterns, which helped to interpret the stolon organization from more general notes on plant organs provided in the literature (mainly from taxonomic descriptions) across all currently known species of the subgenus. Since species descriptions may have been occasionally relied on sparse material available, it cannot be excluded that more stolon types exist. However, for this first comprehensive study on stolon types, the information was sufficient to be included in the PCA. Moreover, we rebut Taylor’s (1989) assumption that bladders randomly occur on internodes of stolons within section Pleiochasia (now Pleiochasia and Lasiocaules), since the PCA showed a significant taxonomic relevance of taxa with and taxa without bladders on internodes.
Stolon types ‘volubilis’, ‘tubulata’, and ‘magna’ were solely found in the respective species. The ‘volubilis’ type stolon was described by Taylor (1989) and Lloyd (1942) as a special rhizoid (anchor stolon). We prefer Merl’s (1915) interpretation of this type being a runner stolon, since the tufts of bladders along the shoot arise on more or less compressed side stems, which can be regarded as secondary shoots. Merl (1915) noticed the presence of runner stolons in U. volubilis with leaves, rhizoids and secondary stolons in addition to bladders arising from short stems. We found this type very occasionally in our material. It was not mentioned by Lloyd (1942) and Taylor (1989).
The ‘correlation group climate’ implies that the stolon type is very strongly related to the taxonomy and to the distribution in regions with similar climate seasonality. Stolon types apparently diverged during evolution of sections and clades in the subgenus, as results of dispersal or vicariance (great climate changes in humidity and temperature) events in Australia in the Miocene and Pliocene (cf. Jobson et al., 2017, 2018b). For instance, rosulate species without horizontal stolons (sections Polypompholyx and Tridentaria, and clade A of Pleiochasia) are confined to the temperate Southwest of Australia, with only U. tenella R. Br. and U. violacea R. Br. reaching the Southeast and Tasmania. Stolon type ‘dichotoma’ is widespread in Australia in both monsoonal tropics and temperate regions, but type ‘uniflora’ is abundant in monsoonal tropics of the Northern Territory and the Kimberley region, except for U. uniflora, which has a wide distribution in temperate zones of the Northeast and Southeast of Australia.
In the PCA, the variable ‘stolon type’ is a dominating factor in the distribution of samples (species) in the plot and on the circumscription of three out of four clusters (Fig. 3). Since the traits ‘stolon type’ and ‘clade’ are very strongly correlated, stolon types have a great taxonomic value. It would, therefore, be helpful to include more detailed information on stolons and branching patterns in future species descriptions. The ‘correlation group phylogeny’ not only contains the traits ‘clade’ and ‘stolon type’ but also ‘leaf form’ and ‘leaf apex’, which shows that the latter two traits are as well taxonomically relevant.
Habit and reproduction
The PCA and linear regressions reveal a ‘correlation group habit’ with the trap morphology being amongst the strongest contributors of clustering in the PCA of subgenus Polypompholyx, and with significant correlations between the traits ‘habit’, ‘leaf form’, ‘leaf apex’, and ‘trap type’. Thus, together with their taxonomic relevance, forms of leaves and leaf apices are strongly correlated to the habit and in turn to the habitat and water levels. This corroborates the findings of Reut et al. (2021), which concluded that in the Lentibulariaceae, narrower leaves are more abundant in submerged to free-floating taxa than in amphibious taxa. Additionally, the current study showed that taxa with traps having filiform dorsal and lateral appendages, and reduced or absent wings, tend to be adapted to submergence or a life in the water column, while traps with ciliate lateral appendages and ciliate wings are often found in amphibious species. This observation confirms results of an earlier phylogenetic study by Reut & Jobson (2010) on 27 species of subgenus Polypomphyolyx.
Furthermore, our analyses demonstrate that the habit of plants within the subgenus corresponds to the traits ‘life cycle’, ‘stolon length’, and ‘plant size’ in the ‘correlation group reproduction’. Emergent to submerged or free-floating species tend to be taller stoloniferous perennials with longer stolons. These correlations fit into the perception that most hydrophytes are perennial with an effective vegetative reproduction (cf. Les & Philbrick, 1993).
The linear regression of ‘life cycle’ and ‘region’ showed a weak correlation between the two traits. About 47% of perennials occur in temperate regions, whilst only 24% of plants from monsoonal regions with long dry periods are perennial. These results are partly in line with Jobson et al. (2017), who demonstrated a significant correlation between climate seasonality and life cycle in subgenus Polypompholyx.
Seedling development
Fleischmann (2012) described two patterns of seedling germination in Genlisea. In one type, no cotyledons are seen on the seedling, and the first primary organs are a leaf and a rhizophyll (trap), the latter with root hairs towards its base. According to Fleischmann (2012), the second seedling type (members of Genlisea sect. Genlisea) expresses two structures that develop into cotyledons. The author refers to similarities of this type of Genlisea germination with the “complex seedling type” of Utricularia species (cf. Lloyd, 1942). However, Lloyd (1942) preferred to use the term “cotyledonoids”, emphasizing that the primary organs are not true cotyledons. Moreover, Kondo et al. (1978) were not in favour of Lloyd’s term “cotyledonoids” since the nature of the first two primary organs would not correspond to cotyledons at all. This view is in line with Kamieński (1877) who noticed that cotyledons remain reduced in the embryo of U. vulgaris L. Moreover, genes typically expressed in embryos and cotyledons were putatively missing in U. gibba L. (Ibarra-Laclette et al., 2013). In this context, it is worth mentioning that some Pinguicula species are anisocotyledonous (i.e. having only one cotyledon), whereas the primary root is reduced (Haccius & Hartl-Baude, 1956). In P. vulgaris L., it seems that after the development of the only cotyledon, the subsequent organs are a foliage leaf and a shoot-borne root (Fig. 10 II in Haccius & Hartl-Baude, 1956). Considering the above mentioned, it can be at least questioned that the two primary organs of seedlings studied on G. pygmaea and U. westonii are cotyledons. However, it may be that the small structure (“scale”) in the seedling of G. pygmaea, which stopped its growth after slightly penetrating the seed testa, could be a reduced cotyledon (or a reduced leaf). This would need to be verified by histochemical investigations and transmission electron microscopy of the embryo. More seedling studies are required to clarify the nature of cotyledons/primary organs (cf. Miranda et al., 2021).
Our results show that the development of a primary leaf and a primary rhizoid (anchor stolon), and the succession and positions of further organs of the U. westonii seedling, are the same pattern as described by Lang (1901) in U. multifida R.Br. and by Lloyd (1937, 1942) in U. tenella, which is in support of the close relationship of sections Tridentaria and Polypompholyx. As demonstrated by Lloyd (1937, 1942), U. dichotoma subsp. monanthos R.W.Jobson seedlings also develop a primary leaf and a primary stolon (a downward growing anchor stolon) followed by a first bladder. The observed germination pattern refers to the “simple seedling type”, which is present in terrestrial Utricularia species including sect. Polypompholyx and U. dichotoma subsp. monanthos (Lloyd 1942). In contrast to U. multifida, U. tenella (cf. Lang, 1901; Lloyd, 1937) and U. westonii, which produce a leaf as forth organ, U. dichotoma subsp. monanthos developed a runner stolon in the material studied by Lloyd (1937).
In summary, the first three organs developing on the seedling of P. vulgaris (after growth of the cotyledon, cf. Haccius & Hartl-Baude, 1956), Genlisea and Utricularia subg. Polypompholyx seem to be a primary foliage leaf, and a first root (P. vulgaris), rhizophyll/trap (Genlisea) or rhizoid/stolon (Polypompholyx), followed by either a leaf or (in Polypompholyx) a trap. By looking at the sequence and position of these organs on the seedling, it could be hypothesized that the first root of Pinguicula, the primary trap of Genlisea, and the primary (anchor) stolon of Polypompholyx are homologous organs. Furthermore, even though the morphologies of (mature) organs look different, they share the functions of anchoring the plant and taking up nutrients from the substrate. These functions may mirror common developmental processes. Indeed, the results of the PCA indicate that, although rhizoids and traps have more in common with shoots and leaves, they still contain developmental processes for roots. Recent genome studies demonstrated that, although many genes underlying root structuring were found to be putatively missing in the genomes of the “rootless” U. gibba and U. vulgaris (Ibarra-Laclette et al. 2013; Renner et al., 2018), some root-related genes remained (Bárta et al., 2015) and may be expressed in e.g. the nutrient uptake by trichomes of bladders (Carretero-Paulet et al., 2015a) or other vegetative organs. Moreover, according to the PCA, the P. gigantea root shares processes with shoots (see also Reut & Płachno, 2020). The reduction of certain anatomical structures and the transfer of carnivory from aerial leaves of Pinguicula to subterranean/submerged traps of Genlisea and Utricularia were probably driven by adaptations to wet and submerged habitats and may have led to evolutionary shifts from root-shoot mosaics to root-shoot-leaf mosaics and shoot-leaf mosaics (cf. Reut & Płachno, 2020; Reut et al., 2021). While the aerial leaves in the Lentibulariaceae have a planar form, the ascidiate traps of Genlisea and Utricularia seem to develop by simple extension and repression of gene expression patterns in the leaf primordium (cf. Lee et al., 2019; Whitewoods et al., 2020; Agrawal et al., 2022).
Process morphology
Already Glück (1906) revealed that the immense plasticity of Utricularia teaches us to give up the classical terms of leaf, shoot and root, which in the end seem to be related to functional and physiological aspects rather than being purely morphologically determined. Goebel (1905) stated: “All attempts that have been made to give a simple definition of ‘caulome’ and of ‘phyllome’ have failed, and this is not surprising seeing that none of the characters upon which they have been based are constant in all of the different cycles of affinity. Plants, it must be remembered, are living things, and the formation of their organs cannot be circumscribed by definitions. What we can say, and what indeed is alone of interest, is this the modifications which the formation of the organs undergo in any one group can only be determined by comparing all their characters”.
In a recent article, Baum (2019) has outlined how plant parts can be grasped by structures, functions, or processes, depending on the scientific context. In the case of the Pinguicula-[Utricularia-Genlisea] ancestor, the root represents a part-as-structure (cf. Baum, 2019), which was abandoned due to the partial loss of genetic factors underlying the development of certain root structures in Utricularia (cf. Ibarra-Laclette et al., 2013; Bárta et al., 2015; Carretero-Paulet et al., 2015a, b; Renner et al., 2018). A part-as-function has the potential to mutate and change its appearance by genetically adapting a new trait (Baum, 2019). In some Polypompholyx taxa, variations in leaf shape, the polymorphism of traps, or the growth of simple stolons from rhizoids may be examples of parts-of-functions, although this would need to be substantiated by further studies between populations and species. Both parts-as-structures and parts-as-functions depend on the basic genetic information, but they do not cover parts or phytomers with their phenotypic differences in an individuum (Baum, 2019). These “variations of the same theme” may be developmental programs governed by gene regulatory networks and influenced by external factors, as explicated by Baum (2019) in the parts-as-processes approach. Interchangeable and homologous organ types on shoots of stolon nodes on the same plant of U. dichotoma sensu lato represent ‘parts-as-processes’ (cf. Reut & Płachno, 2020).
The latest process morphological work on Utricularia with the support of PCA was focussing on the morphological dynamics of populations and vegetative organs of U. dichotoma sensu lato (Reut & Płachno, 2020). Although the current study has a comparable methodological approach, it has a broader scope with the addition of organs from other species of subgenus Polypompholyx and several “typical” roots of living plants. The inclusion of more roots was helpful for the creation of a confidence ellipse of this organ class in the PCA plot, as this was missing in Reut & Płachno (2020). Moreover, the present study has the advantage of being closer to the natural situation, since “theoretical” shoots and leaves (cf. Reut & Płachno, 2020) were replaced by existing shoots and leaves. The general distribution of organs of the Lentibulariaceae and “typical” organs in the morphospace (i.e. the PCA illustration), however, resembles the distribution in the morphospace shown in Reut & Płachno (2020).
Results of the PCA demonstrate that organs reflect mosaics or transitions of developmental processes of root, shoot and leaves, but in each organ the categories with their typical processes are differently weighted. Apart from the “unusual” vegetative organs of Utricularia, we looked at two other “atypical” organs: the compound leaf of Chisocheton tenuis P.F.Stevens with epiphyllous shoots arising along its rachis, and the feathery, chlorophyllous roots of Trapa natans. The position of the C. tenuis leaf in the morphospace suggests that the organ represents a leaf-shoot mosaic (see also Fisher & Rutishauser, 1990) with some more typical shoot characters. There were controversial interpretations of the greenish submerged roots of T. natans in past studies (e.g. Nedukha & Kordyum, 2016; Seago et al., 2016). Our anatomical observation reveal that the organ and its branches develop endogenously from the mother structure, which is typical for roots. The location of the T. natans root close to the barycentre of “typical” roots also indicates that most developmental processes are root related. The presence of chloroplasts in submerged shoots or roots (e.g. in Podostemaceae) is not unusual (cf. Reut et al., 2021) and does not qualify alone for a leaf categorization. Green aerial roots are characteristic for orchids; hence we treated the root of Taeniophyllum biocellatum as “typical” root. This was also reflected by its position in the plot of the PCA, near the barycentre of “typical” roots.
Leaves of U. dichotoma and U. multifida combine many developmental processes of a leaf, although the leaf of U. dichotoma is “more typical” as it is closer to the barycentre of the corresponding ellipse. The two leaves, however, only differ in the axis related position (axillant on the stolon in U. dichotoma, non-axillant/-axillary on a short stem in U. multifida). The distribution of the subterranean organs of Genlisea and Utricularia in the morphospace seems to be along a transect (or morphocline) from the traps of G. repens and U. multifida, and the rhizoid of U. multifida (shoot-leaves mosaics) to the ‘simple stolon’ of U. dichotoma (root-shoot-leaf mosaic), and to the runner stolons of U. dichotoma and U. uniflora, which show similar and strong affinities to both roots and shoots. The root of P. gigantea (root-shoot mosaic) is near the transect, which points to similar developmental profiles of subterranean organs within this group of Lentibulariaceae species. Organs with mixed identities originated from transfers of genetic programs (functions) from a donor structure to a recipient structure (Baum and Donoghue, 2002). Due to the ongoing exchange of genetic information between organisms over time, but also due to epigenetic dynamics, definitions of organs are generally fuzzy as processes are in constant change (cf. Baum, 2019).
With regards to the stolon types in subgenus Polpompholyx, said transect reflects a morphocline of increasing complexity, i.e. from an unbranched rhizoid to a ‘simple stolon’ bearing single bladders to a branched runner stolon. However, there is no evidence that these forms followed transitionary steps in the evolution, since the non-stoloniferous growth form with rhizods in subgenus Polypompholyx is either ancestral or a retrogression from stolonifery (cf. Jobson et al., 2018b). The presence of rhizoids is a plesiomorphic trait in subgenus Polypompholyx, at least on the base of the inflorescence/stem, as it exists in all species except for U. tubulata. Moreover, if the ancestor of subgenus Polypompholyx was stoloniferous, it cannot be conclusively stated whether it had rhizoids on stolon nodes (as prevalent in sect. Pleiochasia) or not (as prevalent in sect. Lasiocaules). It seems likely that the developmental processes leading to single bladders along a stem are the same in ‘simple stolons’ and e.g. runner stolons of type ‘uniflora’, but it cannot be circumstantiated which stolon type evolved first.
Conclusion
We found nine stolon types in the 56 currently known species of subgenus Polypompholyx. Predominant types are runner stolons of type ‘dichotoma’ (with rhizoids on stolon nodes; the prevalent type in sect. Pleiochasia, mainly occurring in the Northeast and/or the Southeast of Australia) and runner stolons of type ‘uniflora’ (without rhizoids on stolon nodes, but with bladders on internodes; the prevalent type in sect. Lasiocaules, mainly distributed in the Northern Territory and/or the Northwest of Australia). According to the PCA performed, stolon types strongly correlate to taxonomic groups and to the regional distribution in relation to climate seasonality. A correspondence of stolon types to life forms, e.g. due to adaptations to periodic or permanent submergence, cannot be demonstrated by our results. It seems that stolon types diversified along with speciation events in Australia, which were partly triggered by drastic climate changes.
In principle, the patterns and organ successions in the seedling development seem to be very similar in Genlisea and Utricularia subg. Polypompholyx. The first organ is always a foliar leaf. The second organ is a trap (Genlisea) or a rhizoid (anchor stolon) (subg. Polypompholyx) followed by a leaf (Genlisea) or a trap (subg. Polypompholyx), and a leaf (Genlisea, and Utricularia sect. Polypompholyx and Tridentaria) or a runner stolon (U. dichotoma susp. monanthos of sect. Pleiochasia). The development of leaves, traps, rhizoids, and stolons in the same positions on the seedling of different taxonomic entities indicate that these organs are homologous. Furthermore, from the perspective of process morphology, their formation involves different combinations of developmental processes for roots, shoots and/or leaves, as statistically demonstrated by PCA. However, within subgenus Polypompholyx, traps and rhizoids seem to be root-shoot-leaf mosaics on one side of a morphocline and with branched runner stolons with a strong root and shoot affinity on the other side. ‘Simple stolons’ bearing single bladders are an intermediate form on this transect. Since the examined subterranean organs of the Lentibulariaceae are in proximal distance in the morphospace, their developmental profiles are not much different. It can be assumed that the trap of the Genlisea-Utricularia ancestor combined developmental processes of a reduced “shoot-like” Pinguicula root with developmental processes of a (maybe modified, ascidiate) Pinguicula leaf (including structures for carnivory).
Partly in accordance with earlier studies, the PCA of several biometric traits across species of subgenus Polypompholyx show that emergent to free-floating species (adapted to submergence) significantly correlate to the perennial life cycle, taller plants, longer stolons, narrower leaves with acute apex, and traps with filiform dorsal and lateral appendages, and reduced or absent wings.
References
Adamec, L. 2020. Biological flora of Central Europe: Utricularia intermedia Hayne, U. ochroleuca R.W. Hartm., U. stygia Thor and U. bremii Heer ex Kölliker. Perspectives in Plant Ecology, Evolution and Systematics 44: e125520.
Adlassnig, W., M. Peroutka, H. Lambers, & I. K. Lichtscheidl. 2005. The roots of carnivorous plants. Plant and Soil 274: 127–140.
Agrawal, A., A. Pareek, & J. Dkhar. 2022. Genetic basis of carnivorous leaf development. Frontiers in Plant Science 12:825289. https://doi.org/10.3389/fpls.2021.825289
Baleeiro, P. C. & R. W. Jobson. 2022. Redescription of Utricularia singeriana and a new species Utricularia baliboongarnang Baleeiro & R.W.Jobson for north-eastern Western Australia. Telopea 25: 63-73. https://doi.org/10.7751/telopea15647
Bárta J., J. D. Stone, J. Pech, D. Sirová, L. Adamec, M. A. Campbell, & H. Štorchová. 2015. The transcriptome of Utricularia vulgaris, a rootless plant with minimalist genome, reveals extreme alternative splicing and only moderate sequence similarity with Utricularia gibba. BMC Plant Biology 15: 78. l0.ll86/sl2870-015-0467-8
Baum, D. A. 2019. Plant parts: Processes, structures, or functions? Gardens’ Bulletin Singapore 71 (Suppl. 2): 225-256.
Baum, D. A. & M. J. Donoghue. 2002. Transference of function, heterotopy, and the evolution of plant development. Pp. 52-69. In: Q. C. B. Cronk, R. A. Bateman, & J. A. Hawkins, (eds), Developmental genetics and plant evolution. Taylor & Francis, London, UK.
Brugger, J. & R. Rutishauser.1989. Bau und Entwicklung landbewohnender Utricularia-Arten. Botanica Helvetica 99: 91–146.
Carretero-Paulet, L., T-H. Chang, P. Librado, E. Ibarra-Laclette, L. Herrera-Estrella, J. Rozas, & V. A. Albert. 2015a. Genome-wide analysis of adaptive molecular evolution in the carnivorous plant Utricularia gibba. Genome Biology and Evolution 7: 444–456.
Carretero-Paulet, L., P. Librado, T-H. Chang, E. Ibarra-Laclette, L. Herrera-Estrella, J. Rozas, & V. A. Albert. 2015b. High gene family turnover rates and gene space adaptation in the compact genome of the carnivorous plant Utricularia gibba. Molecular Biology and Evolution 32: 1284–1295.
Catian, G. & E. Scremin-Dias. 2015. Phenotypic variations in leaf anatomy of Nymphaea gardneriana (Nymphaeaceae) demonstrate its adaptive plasticity. Journal of the Torrey Botanical Society 142: 18–26.
Chomicki, G., Y. Staedler, L. P. R. Bidel, C. Jay-Alleman, J. Schönenberger, & S. S. Renner. 2018. Deciphering the complex architecture of an herb using micro-computed X-ray tomography, with an illustrated discussion on architectural diversity of herbs. Botanical Journal of the Linnean Society 186: 145–157.
Chormanski, T. A. & J. H. Richards. 2012. An architectural model for the bladderwort Utricularia gibba (Lentibulariaceae). Journal of the Torrey Botanical Society 139: 137–148.
Colmer, T. D., A. Winkel, & O. Pedersen. 2011. A perspective on underwater photosynthesis in submerged terrestrial wetland plants. AoB Plants 11: 1–15.
Compton, R. H. 1909. The morphology and anatomy of Utricularia brachiata, Oliver. New Phytologist 8: 117–130.
Crang, R., S. Lyons-Sobaski, & R. Wise. 2018. Plant anatomy. Springer, Cham, Switzerland.
Degtjareva, G., J. Casper, F. Hellwig, & D. Sokoloff. 2004. Seed morphology in the genus Pinguicula (Lentibulariaceae) and its relation to taxonomy and phylogeny. Botanische Jahrbücher für Systematik, Pflanzengeschichte und Pflanzengeographie 125: 431–452.
Degtjareva, G. V., S. J. Casper, F. H. Hellwig, A. R. Schmidt, J. Steiger, & D. D. Sokoloff. 2006. Morphology and nrITS phylogeny of the genus Pinguicula L. (Lentibulariaceae), with special attention to embryo evolution. Plant Biology 8: 778–790.
Fisher, J. B. 2002. Indeterminate leaves of Chisocheton (Meliaceae): survey of structure and development. Botanical Journal of the Linnean Society 139: 207–221.
Fisher, J. B. & R. Rutishauser. 1990. Leaves and epiphyllous shoots in Chisocheton (Meliaceae): a continuum of woody leaf and stem axes. Canadian Journal of Botany 68: 2316-2328.
Fleischmann, A. 2012. Monograph of the genus Genlisea. Redfern Natural History, Poole, Dorset, UK.
Fleischmann, A. 2018. Systematics and evolution of Lentibulariaceae. II. Genlisea. Pp. 81–88. In: A. M. Ellison & L. Adamec, (eds.), Carnivorous plants: physiology, ecology, and evolution. Oxford University Press, New York, USA. https://doi.org/10.1093/oso/9780198779841.003.0007
Fleischmann, A. & A. Roccia. 2018. Systematics and evolution of Lentibulariaceae. I. Pinguicula. Pp. 70–80. In: A. M. Ellison & L. Adamec, (eds.), Carnivorous plants: physiology, ecology, and evolution. Oxford University Press, New York, USA. https://doi.org/10.1093/oso/9780198779841.003.0006
Fleischmann, A., B. Schäferhoff, G. Heubl, F. Rivadavia, W. Barthlott, & K. F. Müller. 2010. Phylogenetics and character evolution in the carnivorous plant genus Genlisea A. St.-Hil. (Lentibulariaceae). Molecular Phylogenetics and Evolution 56: 768-783.
Ganong, W.F. 1901. The cardinal principles of morphology. Botanical Gazette 31: 426-434.
Ganong, W.F. 1913. The living plant; a description and interpretation of its functions and structure. Holt, New York, USA.
Gassin, R. J. 1993. Utricularia beaugleholei (Lentibulariaceae; subgenus Utricularia: section Pleiochasia), a new species from Sout-eastern Australia. Muelleria 87: 37-42.
Gleissberg, S. 2004. Comparative analysis of leaf shape development in Eschscholzia californica and other Papaveraceae-Eschscholzioideae. American Journal of Botany 91: 306–312.
Glück, H. 1906. Biologische und morphologische Untersuchungen über Wasser- und Sumpfgewächse. 2. Teil. Untersuchungen über die mitteleuropäischen Utricularia-Arten, über die Turionenbildung bei Wasserpflanzen, sowie über Ceratophyllum. G. Fischer, Jena, Germany.
Goebel, K. 1891. Pflanzenbiologische Schilderungen. Pp. 121–160. Elwert, Marburg, Germany.
Goebel, K. 1905. Organography of plants. Part 2. engl. I. B. Balfour, (ed.). At the Clarendon Press, Oxford, UK.
Gomes Rodrigues, F., N. Franco Marulanda, S. R. Silva, B. J. Płachno, L. Adamec, & V. F. O. Miranda. 2017. Phylogeny of the 'orchid-like' bladderworts (gen. Utricularia sect. Orchidioides and Iperua: Lentibulariaceae) with remarks on the stolon-tuber system. Annals of Botany 120: 709–723. https://doi.org/10.1093/aob/mcx056
Greilhuber, J., T. Borsch, K. Müller, A. Worberg, S. Porembski, & W. Barthlott. 2006. Smallest angiosperm genomes found in Lentibulariaceae, with chromosomes of bacterial size. Plant Biology 8: 770–777.
Grob, V., P. Moline, E. Pfeifer, A. R. Novelo, & R. Rutishauser. 2006. Developmental morphology of branching flowers in Nymphaea prolifera. Journal of Plant Research 119: 561–570. https://doi.org/10.1007/s10265-006-0021-8
Guisande, C., C. Granado-Lorencio, C. Andrade-Sossa, & S. R. Duque. 2007. Bladderworts. Functional Plant Science and Biotechnology 1: 58–68.
Gupta, M. D. & U. Nath. 2015. Divergence in patterns of leaf growth polarity is associated with the expression divergence of miR396. The Plant Cell: 2785–2799.https://doi.org/10.1105/tpc.15.00196
Haccius, B. & E. Hartl-Baude. 1956. Embryologische und histologische Studien an “monokotylen Dikotylen” II. Pinguicula vulgaris L. und Pinguicula alpina L. Österreichische Botanische Zeitschrift 103: 567-587.
Hidalgo, O., S. Garcia, T. Garnatje, M. Mumbrú, A. Patterson, J. Vigo, & J. Vallès. 2015. Genome size in aquatic and wetland plants: fitting with the large genome constraint hypothesis with a few relevant exceptions. Plant Systematics and Evolution 301: 1927–1936. https://doi.org/10.1007/s00606-015-1205-2
Ibarra-Laclette, E., E. Lyons, G. Hernández-Guzmán, C. A. Pérez-Torres, L. Carretero-Paulet, T-H. Chang, T. Lan, A. J. Welch, M. J. Abraham Juárez, J. Simpson, A. Fernández-Cortés, M. Arteaga-Vázquez, E. Góngora-Castillo, G. Acevedo-Hernández, S. C. Schuster, H. Himmelbauer, A. E. Minoche, S. Xu, M. Lynch, A. Oropeza-Aburto, S. A. Cervantes-Pérez, M. de Jesús Ortega-Estrada, J. I. Cervantes-Luevano, T. P. Michael, T. Mockler, D. Bryant, A. Herrera-Estrella, V. A. Albert, & L. Herrera-Estrella. 2013. Architecture and evolution of a minute plant genome. Nature 498: 94–98.
Ikeuchi, M., K. Tatematsu, T. Yamaguchi, K. Okada, & H. Tsukaya. 2013. Precocious progression of tissue maturation instructs basipetal initiation of leaflets in Chelidonium majus subsp. asiaticum (Papaveraceae). American Journal of Botany 100: 1116–1126.
Jeune, B. & R. Sattler. 1992. Multivariate analysis in process morphology of plants. Journal of Theoretical Biology 156: 147-167.
Jeune, B., D. Barabé, & C. Lacroix. 2006. Classical and dynamic morphology: toward a synthesis through the space of forms. Acta Biotheoretica 54: 277-293. https://doi.org/10.1007/s10441-007-9007-8
Jobson, R. W. 2012. A new species of Utricularia (Lentibulariaceae) from northern Queensland, Australia. Telopea 14: 49–57. https://doi.org/10.7751/telopea2012008
Jobson, R. W. 2013. Five new species of Utricularia (Lentibulariaceae) from Australia. Telopea 15: 127–142. https://doi.org/10.7751/telopea2013017
Jobson, R. W. & P. C. Baleeiro. 2015. Two new species of Utricularia (Lentibulariaceae) from the North West region of Western Australia. Telopea 18: 201–208. https://doi.org/10.7751/telopea8894
Jobson, R. W. & P. C. Baleeiro. 2020. Radiations of fairy-aprons (Utricularia dichotoma, Lentibulariaceae) in Australia and New Zealand: molecular evidence and proposal of new subspecies Australian Systematic Botany, 33: 278–310. https://doi.org/10.1071/SB19003
Jobson, R. W. & W. Cherry. 2020. Utricularia gaagudju, a new species for the Northern Territory, and a recircumscription of U. kimberleyensis C.A.Gardner. Telopea 23: 61-68. https://doi.org/10.7751/telopea14168
Jobson, R. W., J. Playford, K. M. Cameron, & V. A. Albert. 2003. Molecular phylogenetics of Lentibulariaceae inferred from plastid rps16 intron and trnL-F DNA sequences: implications for character evolution and biogeography. Systematic Botany 28: 157–171.
Jobson, R. W., P. C. Baleeiro, & M. S. Reut. 2017. Molecular phylogeny of subgenus Polypompholyx (Utricularia; Lentibulariaceae) based on three plastid markers: diversification and proposal for a new section. Australian Systematic Botany 30: 259–278.
Jobson, R. W., P. C. Baleeiro, & M. D. Barrett. 2018a. Six new species of Utricularia (Lentibulariaceae) from Northern Australia. Telopea 21: 57–77. https://dx.doi.org/https://doi.org/10.7751/telopea12630
Jobson, R. W., P. C. Baleeiro, & C. Guisande. 2018b. Systematics and evolution of Lentibulariaceae: III. Utricularia. Pp. 89–104. In: A. M. Ellison & L. Adamec, (eds.), Carnivorous plants: physiology, ecology, and evolution. Oxford University Press, New York, USA. https://doi.org/10.1093/oso/9780198779841.003.0008
Juniper, B. E., R. J. Robins, & D. M. Joel. 1989. The carnivorous plants. Academic Press, London, UK.
Kamieński, F. 1876. Porównawcze badania nad wzrostem pływaczów (Utricularia). [Comparative research on the growth of bladderworts (Utricularia)]. Rozprawy i Sprawozdania z Posiedzeń Wydziału Matematyczno-Przyrodniczego Akademii Umiejętności 3: 210–240.
Kamieński, F. 1877. Vergleichende Untersuchungen über die Entwicklungsgeschichte der Utricularien. Botanische Zeitung (Berlin) 35: 761-775.
Kirchoff, B. K., E. Pfeifer, & R. Rutishauser. 2008. Plant structure ontology: how should we label plant structures with doubtful or mixed identities? Zootaxa 1950: 103–122.
Kondo, K., M. Segawa, & K. Nehira. 1978. Anatomical studies on seeds and seedlings of some Utricularia (Lentibulariaceae). Brittonia 30: 89–95.
Krähmer, H. & P. Baur. 2013. Weed anatomy. Wiley-Blackwell, Chichester, UK.
Lang, F. X. 1901. Untersuchungen über Morphologie, Anatomie und Samenentwicklung von Polypompholyx und Byblis gigantea. Flora 88: 149-206.
Lee, K. J. I., C. Bushell, Y. Koide, J. A. Fozard, C. Piao, M. Yu, J. Newman, C. Whitewoods, J. Avondo, R. Kennaway, A. F. M. Marée, M. Cui, & E. Coen. 2019. Shaping of a threedimensional carnivorous trap through modulation of a planar growth mechanism. PLoS Biology 17(10):e3000427. https://doi.org/10.1371/journal.pbio.3000427
Legendre, L. 2000. The genus Pinguicula L. (Lentibulariaceae): an overview. Acta Botanica Gallica 147: 77-95. https://doi.org/10.1080/12538078.2000.10515837
Les, D. H. & C. T. Philbrick. 1993. Studies of hybridization and chromosome number variation in aquatic angiosperms: evolutionary implications. Aquatic Botany 44: 181–228.
Lloyd, F. E. 1937. Utricularia: Its development from the seed. Journal of South African Botany 3: 155-164.
Lloyd, F. E. 1942. The carnivorous plants. Chronica Botanica Co., Waltham, Mass., USA.
Lovett-Doust, J., L. Lovett-Doust, & A.T. Growth. 1990. The biology of Canadian weeds. 95. Ranunculus repens. Canadian Journal of Plant Science 70: 1123-1141.
Lowrie, A. 1998. A new species of Utricularia (Lentibulariaceae) from the south-west of Western Australia. Nuytsia 12: 37–41.
Lowrie, A. 2002. Utricularia petertaylorii (Lentibulariaceae), a new species from the south west of Western Australia. Nuytsia 14: 405–410.
McAllister, H. A. 1999. Lysimachia punctata L. and L. verticillaris Sprengel (Primulaceae) naturalised in the British Isles. Watsonia 22: 279-281.
Merl, E. M. 1915. Beiträge zur Kenntnis der Utricularien und Genliseen. Flora 108: 127-200.
Metsalu, T. & J. Vilo. 2015. Clustvis: a web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Research 43: W566–W570. https://doi.org/10.1093/nar/gkv468
Miranda, V. F. O., S. R. Silva, M. S. Reut, H. Dolsan, P. Stolarczyk, R. Rutishauser, & B. J. Płachno. 2021. A historical perspective of bladderworts (Utricularia): traps, carnivory and body architecture. Plants 10: 2656. https://doi.org/10.3390/plants10122656.
Mohammadi Shahrestani, M, M. Beygom Faghir, & M. Assadi. 2020. Comparative anatomical studies in relation to taxonomy of Sedum s.l. (Crassulaceae) in Iran. Turkish Journal of Botany 44: 281-294.
Müller, K. & T. Borsch. 2005. Phylogenetics of Utricularia (Lentibulariaceae) and molecular evolution of the trnK intron in a lineage with high substitutional rates. Plant Systematics and Evolution 250: 39–67. https://doi.org/10.1007/s00606-004-0224-1
Nakayama, H., N. R. Sinha, & S. Kimura. 2017. How do plants and phytohormones accomplish heterophylly, leaf phenotypic plasticity, in response to environmental cues. Frontiers in Plant Science 8: 1717. https://doi.org/10.3389/fpls.2017.01717
Nedukha, O. M. & E. Kordyum. 2016. The plasticity of anatomical structure and cell wall lignin in Trapa natans adaptation to nature flooding. Annals of the Romanian Society for Cell Biology 21: 27-34.
Nurfadilah, S., N. D. Yulia, & E. E. Ariyanti. 2016. Morphology, anatomy, and mycorrhizal fungi colonisation in roots of epiphytic orchids of Sempu Island, East Java, Indonesia. Biodiversitas 17: 592-603.
Pinto, T. M., M. do Rosário Anjos, N. M. Martins, J. Gomes-Laranjo, J. Ferreira-Cardoso, & F. Peixo. 2011. Structural analysis of Castanea sativa Mill. leaves from different regions in the tree top. Brazilian Archives of Biology and Technology 54: 117–124.
Płachno, B. J. & P. Świątek. 2010. Unusual embryo structure in viviparous Utricularia nelumbifolia, with remarks on embryo evolution in genus Utricularia. Protoplasma 239: 69–80. https://doi.org/10.1007/s00709-009-0084-1
Płachno, B. J., K. Adamus, J. Faber, & J. Kozłowski. 2005. Feeding behaviour of carnivorous Genlisea plants in the laboratory. Acta Botanica Gallica 152: 159–164.
Płachno B. J., L. Adamec, & I. Kamińska. 2014. Relationship between trap anatomy and function in Australian carnivorous bladderworts (Utricularia) of the subgenus Polypompholyx. Aquatic Botany 120: 290–296.
Płachno, B. J., I. Kamińska, L. Adamec, & P. Świątek. 2017. Vascular tissue in traps of Australian carnivorous bladderworts (Utricularia) of the subgenus Polypompholyx. Aquatic Botany 142: 25–31.
Płachno, B. J., P. Świątek, V. F. O. Miranda, & P. Stolarczyk. 2019. The structure and occurrence of a velum in Utricularia traps (Lentibulariaceae). Frontiers in Plant Science 10: 302. https://doi.org/10.3389/fpls.2019.00302
Poppinga, S., C. Weisskopf, A. S. Westermeier, T. Masselter, & T. Speck. 2016. Fastest predators in the plant kingdom: functional morphology and biomechanics of suction traps found in the largest genus of carnivorous plants. AoB Plants 8: plv140. https://doi.org/10.1093/aobpla/plv140
Renner, T., T. Lan, K. M. Farr, E. lbarra-Laclette, L. Herrera-Estrella, S. C. Schuster, M. Hasebe, K. Fukushima, & V. A. Albert. 2018. Carnivorous plant genomes. Pp. 135–153. In: A. M. Ellison & L. Adamec, (eds.), Carnivorous plants: physiology, ecology, and evolution. Oxford University Press, New York, USA. https://doi.org/10.1093/oso/9780198779841.003.0011
Reut, M. S. 1993a. Trap structure of the carnivorous plant Genlisea (Lentibulariaceae). Botanica Helvetica 103: 101-111.
Reut, M. S. 1993b. Genlisea St.-Hil. (Lentibulariaceae) – Morphologie und Biologie einer karnivoren Pflanze. MSc Thesis, Institut für Systematische Botanik, Universität Zürich, Switzerland.
Reut, M. S. & R. W. Jobson. 2010. A phylogenetic study of subgenus Polypompholyx: a parallel radiation of Utricularia (Lentibulariaceae) throughout Australasia. Australian Systematic Botany 23: 152–161. https://doi.org/10.1071/SB09054
Reut, M. S. & B. J. Płachno. 2020. Unusual developmental morphology and anatomy of vegetative organs in Utricularia dichotoma – leaf, shoot and root dynamics. Protoplasma 257: 371-390. https://doi.org/10.1007/s00709-019-01443-6
Reut, M. S., P. Świątek, V. F. O. Miranda, & B. J. Płachno. 2021. Living between land and water – structural and functional adaptations in bladderworts. Plant and Soil 464: 237-255. https://doi.org/10.1007/s11104-021-04929-6
Rutishauser, R. 2016. Evolution of unusual morphologies in Lentibulariaceae (bladderworts and allies) and Podostemaceae (river-weeds): a pictorial report at the interface of developmental biology and morphological diversification. Annals of Botany 117: 811–832. https://doi.org/10.1093/aob/mcv172
Rutishauser, R. 2020. EvoDevo: Past and future of continuum and process plant morphology. Philosophies 5: 41. https://doi.org/10.3390/philosophies5040041
Rutishauser, R & R. Sattler. 1989. Complementarity and heuristic value of contrasting models in structural botany. III. Case study on shoot-like “leaves” and leaf-like “shoots” in Utricularia macrorhiza and U. purpurea (Lentibulariaceae). Botanische Jahrbücher für Systematik 111: 121–137.
Rutishauser, R & B. Isler. 2001. Developmental genetics and morphological evolution of flowering plants, especially bladderworts (Utricularia): Fuzzy Arberian Morphology complements classical morphology. Annals of Botany 88: 1173–1202.
Sattler, R & B. Jeune. 1992. Multivariate analysis confirms the continuum view of plant form. Annals of Botany 69: 249-262.
Sattler, R., D. Luckert, & R. Rutishauser. 1988. Symmetry in plants: phyllode and stipule development in Acacia longipedunculata. Canadian Journal of Botany 66: 1270-1284.
Scavone, O. & S. Panizza. 1973. Sobre a morfologia e anatomia do Nasturtium officinale E. Brown. Boletin de Botânica 1: 117-148.
Schweingruber, F. H., A. Borner, & E-D. Schulze. 2011. Atlas of stem anatomy in herbs, shrubs and trees, Vol. 1. Springer-Verlag, Berlin, Germany.
Sculthorpe, C. D. 1967. The biology of aquatic vascular plants. Edward Arnold Ltd., London, UK.
Seago, J. L., Jr. 2020. Revisiting the occurrence and evidence of endodermis in angiosperm shoots. Flora 273: 151709. https://doi.org/10.1016/j.flora.2020.151709
Seago, J., L, Jr, W. B. Eyres, & M. Volny. 2016. Selected structural features of the riverine plants, Trapa natans (Lythraceae) and Justicia americana (Acanthaceae). Pp. 251–269. In: D. Bucur, (ed.), River Basin Management. Intech, Rijeka, Croatia. https://doi.org/10.5772/63709
Silva, S. R., R. Gibson, L. Adamec, Y. Domínguez, & V. F. O. Miranda. 2018. Molecular phylogeny of bladderworts: A wide approach of Utricularia (Lentibulariaceae) species relationships based on six plastidial and nuclear DNA sequences. Molecular Phylogenetics and Evolution 118: 244–264. https://doi.org/10.1016/j.ympev.2017.10.010
Sinjushin, A. A. 2018. Revisiting the floral structure and ontogeny of Trapa natans L. (Lythraceae). Wulfenia 25: 57– 69.
Studnička, M. 2011. Surprising phenomena in the life strategy of Utricularia cornigera in Brazil. Thaiszia Journal of Botany 21: 37-43.
Sudhakaran, M.V. 2017. Botanical pharmacognosy of Centella asiatica (Linn.) Urban. Pharmacognosy Journal 9: 546-55.
Taylor, P. 1989. The genus Utricularia — a taxonomic monograph. Kew Bulletin Additional Series 14: 1–735.
Troll, W. & H. Dietz. 1954. Morphologische und histogenetische Untersuchungen an Utricularia-Arten. Oesterreichische Botanische Zeitung 101: 165–207.
Tsukaya, H. 2014. Comparative leaf development in angiosperms. Current Opinion in Plant Biology 17: 103–109. https://doi.org/10.1016/j.pbi.2013.11.012
Vaidya, M. S. & K. Dalvi. 2020. Anatomical studies of the medicinally important plant Bauhinia pupurea Linn. Journal of Pharmacognosy and Phytochemistry: 2103–2106.
Veleba, A., P. Bureš, L. Adamec, P. Šmarda, I. Lipnerová, & L. Horová. 2014. Genome size and genomic GC content evolution in the miniature genome-sized family. New Phytologist 203: 22–28.
Voesenek, L. A. C. J., T. D. Colmer, R. Pierik, F. F. Millenaar, & A. J. M. Peeters. 2006. How plants cope with complete submergence. New Phytologist 170: 213–226.
Wakabayashi, H. 2010. Utricularia linearis (Lentibulariaceae), a new species from the Howard Springs, Northern Territory, Australia. The Journal of Insectivorous Plant Society 61: 88–92.
Wang, J.-C., B-P. Pan, & D. C. Albach. 2016. Evolution of morphological and climatic adaptations in Veronica L. (Plantaginaceae). PeerJ 4:e2333. https://doi.org/10.7717/peerj.2333
Warming, E. 1874. Bidrag til Kundskapen om Lentibulariaceae. Videnskabelige Meddelelser fra den naturhistoriske Forening i Kjöbenhavn 3-7: 33–45.
Westermeier, A. S, A. Fleischmann, K. Müller, B. Schäferhoff, C. Rubach, T. Speck, & S. Poppinga. 2017. Trap diversity and character evolution in carnivorous bladderworts (Utricularia, Lentibulariaceae). Scientific Reports 7: 12052. https://doi.org/10.1038/s41598-017-12324-4
Wetzel, R. G. 1988. Water as an environment for plant life. Pp. 1–30. In: Vegetation of Inland Waters, J. J. Symoens, (ed.). Kluwer, Dordrecht, The Netherlands.
Whitewoods, C. D., B. Goncalves, J. Cheng, M. Cui, R. Kennaway, K. Lee, C. Bushell, M. Yu, C. Piao, & E. Coen. 2020. Evolution of carnivorous traps from planar leaves through simple shifts in gene expression. Science 367: 91-96. https://doi.org/10.1126/science.aay5433
Acknowledgements
This research was partially supported financially by the Ministry of Science and Higher Education of Poland as part of the statutory activities of the Department of Plant Cytology and Embryology, Institute of Botany, Faculty of Biology, Jagiellonian University in Kraków (N18/DBS/000002).
The article is dedicated to the Polish Botanical Society on the occasion of the 100th anniversary of its activity. We are cordially grateful to Lubomír Adamec (Institute of Botany of the Czech Academy of Sciences, Třeboň, Czech Republic), Kamil Pásek (Best Carnivorous Plants Store, Ostrava, Czech Republic), Nigel Hewitt-Cooper (UK, www.hccarnivorousplants.co.uk), and Nook Vosse (The Netherlands, https://vosserareplants.com) for material from their collections, and to Marco Pezzotta, Italy for kindly providing seedlings for this study. We also thank the staff of the Botanic Garden of Jagiellonian University in Kraków, Poland for Trapa natans material. We sincerely thank two anonymous reviewers for their insightful comments and suggestions that helped to improve the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no conflicts of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Reut, M.S., Płachno, B.J. Development, Diversity and Dynamics of Plant Architecture in Utricularia subgenus Polypompholyx – Towards Understanding Evolutionary Processes in the Lentibulariaceae. Bot. Rev. 89, 201–236 (2023). https://doi.org/10.1007/s12229-022-09283-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12229-022-09283-5