Abstract
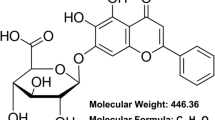
NLRP3 inflammasome is very important in liver diseases and is intimately linked to oxidative stress. Quercetin is a significant antioxidant and anti-inflammatory biological molecule. The aim of current study was to investigate the effects of quercetin on oxidative stress and NLRP3 and caspase-1 proteins involved in sterile inflammation induced by H2O2 in clone-9 cells. MTT, DCF-DA assays, immunocytochemical and fluorescence staining methods were used. After 24 h incubation with H2O2, the IC50 was found to be 278 μM in clone-9 cells. The most effective dose of quercetin beside H2O2-associated damage was found to be 15 μM. It was shown that 278 μM of H2O2 caused pyknosis in cell nuclei, while 15 μM of quercetin protected the cells from the pyknosis. Quercetin pretreatment was shown to reduce H2O2-induced ROS production significantly (p < 0.05). It was found that NLRP3 and caspase-1 immunoreactivity were increased in H2O2-treated groups and quercetin pretreatment reduced the expression of these proteins. According to the results, quercetin reduceses sterile inflammation proteins and H2O2-induced oxidative stress in clone-9 liver cells. Quercetin may be therapeutic in the treatment of liver and NLRP3 inflammasome based diseases. Quercetin can also be taken with food for prophylactic purposes.







Similar content being viewed by others
References
Aherne SA, O’Brien NM (1999) Protection by the flavonoids myricetin, quercetin, and rutin against hydrogen peroxide-induced DNA damage in Caco-2 and Hep G2 cells. Nutr Cancer 34(2):160–166
Ahsan MR, Islam KM, Bulbul IJ, Musaddik MA, Haque E (2009) Hepatoprotective activity of methanol extract of some medicinal plants against carbon tetrachloride-induced hepatotoxicity in rats. Eur J Sci Res 37(2):302–310
Atashpour S, Fouladdel S, Movahhed TK, Barzegar E, Ghahremani MH, Ostad SN, Azizi E (2015) Quercetin induces cell cycle arrest and apoptosis in CD133+ cancer stem cells of human colorectal HT29 cancer cell line and enhances anticancer effects of doxorubicin. Iran J Basic Med Sci 18(7):635–643
Banihosseini SZ, Novin MG, Nazarian H, Piryaei A, Parvardeh S, Eini F (2018) Quercetin improves developmental competence of mouse oocytes by reducing oxidative stress during in vitro maturation. Ann Anim Sci 18(1):87–98
Begum G, Dastagir G, Rauf A, Bawazeer S, Rahman KU, Ramadan MF (2020) Pharmacognostic characteristics and phytochemical profile of various parts of Parthenium hysterophorus. Rend Fis Acc Lincei 31(3):853–872
Çelik N, Vurmaz A, Kahraman A (2017) Protective effect of quercetin on homocysteine-induced oxidative stress. Nutrition 33:291–296
Cichoż-Lach H, Michalak A (2014) Oxidative stress as a crucial factor in liver diseases. World J Gastroenterol 20(25):8082–8091. https://doi.org/10.3748/wjg.v20.i25.8082
Coskun O, Kanter M, Korkmaz A, Oter S (2005) Quercetin, a flavonoid antioxidant, prevents and protects streptozotocin-induced oxidative stress and beta-cell damage in rat pancreas. Pharmacol Res 51(2):117–123. https://doi.org/10.1016/j.phrs.2004.06.002
Duranti G, Ceci R, Patrizio F, Sgro P, Di Luigi L, Sabatini S, Bazzucchi I (2018) Chronic consumption of quercetin reduces erythrocytes oxidative damage: Evaluation at resting and after eccentric exercise in humans. Nutr Res 50:73–81. https://doi.org/10.1016/j.nutres.2017.12.002
Durazzo A, Nazhand A, Lucarini M, Silva AM, Souto SB, Guerra F, Santini A (2021) Astragalus (Astragalus membranaceus Bunge): botanical, geographical, and historical aspects to pharmaceutical components and beneficial role. Rend Fis Acc Lincei 32:625–642
Fedchenko N, Reifenrath J (2014) Different approaches for interpretation and reporting of immunohistochemistry analysis results in the bone tissue—a review. Diagn Pathol 9(1):1–12
Gao F-J, Zhang S-H, Xu P, Yang B-Q, Zhang R, Cheng Y, Chen J-Y (2017) Quercetin declines apoptosis, ameliorates mitochondrial function and improves retinal ganglion cell survival and function in in vivo model of glaucoma in rat and retinal ganglion cell culture in vitro. Front Mol Neurosci 10:285
Herranz-López M, Borrás-Linares I, Olivares-Vicente M, Gálvez J, Segura-Carretero A, Micol V (2017) Correlation between the cellular metabolism of quercetin and its glucuronide metabolite and oxidative stress in hypertrophied 3T3-L1 adipocytes. Phytomedicine 25:25–28
Kalantari H, Foruozandeh H, Khodayar MJ, Siahpoosh A, Saki N, Kheradmand P (2018) Antioxidant and hepatoprotective effects of Capparis spinosa L. fractions and Quercetin on tert-butyl hydroperoxide- induced acute liver damage in mice. J Tradit Complement Med 8(1):120–127. https://doi.org/10.1016/j.jtcme.2017.04.010
Kim GN, Jang HD (2009) Protective mechanism of quercetin and rutin using glutathione metabolism on H2O2-induced oxidative stress in HepG2 Cells. Ann N Y Acad Sci 1171(1):530–537
Koyama Y, Brenner DA (2017) Liver inflammation and fibrosis. J Clin Invest 127(1):55–64
Kurdi A, Hassan K, Venkataraman B, Rajesh M (2018) Nootkatone confers hepatoprotective and anti-fibrotic actions in a murine model of liver fibrosis by suppressing oxidative stress, inflammation, and apoptosis. J Biochem Mol Toxicol 32(2):1–9. https://doi.org/10.1002/jbt.22017
Lesjak M, Beara I, Simin N, Pintać D, Majkić T, Bekvalac K, Mimica-Dukić D (2018) Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J Funct Foods 40:68–75
Li S, Tan H-Y, Wang N, Zhang Z-J, Lao L, Wong C-W, Feng Y (2015) The role of oxidative stress and antioxidants in liver diseases. Int J Mol Sci 16(11):26087–26124. https://doi.org/10.3390/ijms161125942
Lin J, Zhou W (2018) Role of quercetin in the physicochemical properties, antioxidant and antiglycation activities of bread. J Funct Foods 40:299–306
Margina D, Gradinaru D, Manda G, Neagoe I, Ilie M (2013) Membranar effects exerted in vitro by polyphenols—quercetin, epigallocatechin gallate and curcumin—on HUVEC and Jurkat cells, relevant for diabetes mellitus. Food Chem Toxicol 61:86–93. https://doi.org/10.1016/j.fct.2013.02.046
Özcan O, Erdal H, Çakırca G, Yönden Z (2015) Oksidatif stres ve hücre içi lipit, protein ve DNA yapıları üzerine etkileri. J Clin Exp Invest 6(3):331–336
Patusco R, Zelig R, Parker A (2018) Vitamin E supplementation in pediatric nonalcoholic fatty liver disease. Top Clin Nutr 33(1):50–68
Ramos AA, Lima CF, Pereira ML, Fernandes-Ferreira M, Pereira-Wilson C (2008) Antigenotoxic effects of quercetin, rutin and ursolic acid on HepG2 cells: evaluation by the comet assay. Toxicol Lett 177(1):66–73. https://doi.org/10.1016/j.toxlet.2008.01.001
Sekaran S, Kandaswamy S, Gunasekaran K, Perumal E, AfsarBasha FY, Madhan Mohan BJ, Jagadeesan A (2012) Protective role of quercetin on polychlorinated biphenyls (Aroclor-1254) induced oxidative stress and apoptosis in liver of adult male rats. J Biochem Mol Toxicol 26(12):522–532. https://doi.org/10.1002/jbt.21466
Selvakumar K, Bavithra S, Suganya S, Ahmad Bhat F, Krishnamoorthy G, Arunakaran J (2013) Effect of quercetin on haematobiochemical and histological changes in the liver of polychlorined biphenyls-induced adult male wistar rats. J Biomark 2013:1–12. https://doi.org/10.1155/2013/960125
Seufi AM, Ibrahim SS, Elmaghraby TK, Hafez EE (2009) Preventive effect of the flavonoid, quercetin, on hepatic cancer in rats via oxidant/antioxidant activity: molecular and histological evidences. J Exp Clin Cancer Res 28(1):80–80. https://doi.org/10.1186/1756-9966-28-80
Shen H, Kreisel D, Goldstein DR (2013) Processes of sterile inflammation. J Immunol 191(6):2857–2863
Son YO, Jang YS, Heo JS, Chung WT, Choi KC, Lee JC (2009) Apoptosis-inducing factor plays a critical role in caspase-independent, pyknotic cell death in hydrogen peroxide-exposed cells. Apoptosis 14(6):796–808. https://doi.org/10.1007/s10495-009-0353-7
Webb C, Twedt D (2008) Oxidative stress and liver disease. Vet Clin North Am Small Anim Pract 38(1):125–135. https://doi.org/10.1016/j.cvsm.2007.10.001
Weng CJ, Chen MJ, Yeh CT, Yen GC (2011) Hepatoprotection of quercetin against oxidative stress by induction of metallothionein expression through activating MAPK and PI3K pathways and enhancing Nrf2 DNA-binding activity. N Biotechnol 28(6):767–777. https://doi.org/10.1016/j.nbt.2011.05.003
Woolbright BL, Jaeschke H (2017) The impact of sterile inflammation in acute liver injury. J Clin Transl Res 3(Suppl 1):170–188
Wu J, Xu X, Li Y, Kou J, Huang F, Liu B, Liu K (2014) Quercetin, luteolin and epigallocatechin gallate alleviate TXNIP and NLRP3-mediated inflammation and apoptosis with regulation of AMPK in endothelial cells. Eur J Pharmacol 745:59–68. https://doi.org/10.1016/j.ejphar.2014.09.046
Xiao L, Luo G, Tang Y, Yao P (2018) Quercetin and iron metabolism: what we know and what we need to know. Food Chem Toxicol 114:190–203. https://doi.org/10.1016/j.fct.2018.02.022
Yang CF, Shen HM, Ong CN (1999) Protective effect of ebselen against hydrogen peroxide-induced cytotoxicity and DNA damage in HepG2 cells. Biochem Pharmacol 57(3):273–279
Zeng X, Yang J, Hu O, Huang J, Ran L, Chen M, Mi M (2018) Dihydromyricetin ameliorates nonalcoholic fatty liver disease by improving mitochondrial respiratory capacity and redox homeostasis through modulation of SIRT3 signaling. Antioxid Redox Signal. https://doi.org/10.1089/ars.2017.7172
Zhang X, Ji R, Sun H, Peng J, Ma X, Wang C, Jin Y (2018) Scutellarin ameliorates nonalcoholic fatty liver disease through the PPARgamma/PGC-1alpha-Nrf2 pathway. Free Radic Res 52(2):198–211. https://doi.org/10.1080/10715762.2017.1422602
Zhong Z, Zhai Y, Liang S, Mori Y, Han R, Sutterwala FS, Qiao L (2013) TRPM2 links oxidative stress to NLRP3 inflammasome activation. Nat Commun 4:1611. https://doi.org/10.1038/ncomms2608
Zhou X, Yang G, Davis CA, Doi SQ, Hirszel P, Wingo CS, Agarwal A (2004) Hydrogen peroxide mediates FK506-induced cytotoxicity in renal cells. Kidney Int 65(1):139–147. https://doi.org/10.1111/j.1523-1755.2004.00380.x
Zishan A, Anwar S, Shiwali S (2017) Evaluation of in vitro antioxidant activity, HPLC and GC–MS analysis along with chemoprofiling of Decalepis arayalpathra: a critically endangered plant of Western Ghats, India. Rend Fis Acc Lincei 28(4):711–720
Zou B, Xiao G, Xu Y, Wu J, Yu Y, Fu M (2018) Persimmon vinegar polyphenols protect against hydrogen peroxide-induced cellular oxidative stress via Nrf2 signalling pathway. Food Chem 255:23–30. https://doi.org/10.1016/j.foodchem.2018.02.028
Author information
Authors and Affiliations
Contributions
All authors participated in this study. ES designed the study. ES, SDP, VS, and ATK performed assays. ES drafted the manuscript. ES, SDP, VS, and ATK: critical revision of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Our research does not need ethical approval.
Human and animal rights
This article does not contain any studies with human or animal subjects.
Consent for publication
All authors confirmed the publication of the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sahin, E., Dabagoglu Psav, S., Sahinturk, V. et al. Quercetin decreases sterile inflammation proteins NLRP3 and caspase 1 in clone-9 cell line damaged by hydrogen peroxide. Rend. Fis. Acc. Lincei 32, 911–919 (2021). https://doi.org/10.1007/s12210-021-01031-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12210-021-01031-y