Abstract
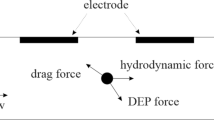
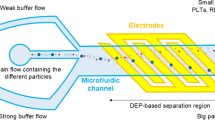
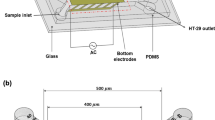
This numerical study proposes a cell sorting technique based on dielectrophoresis (DEP) in a microfluidic chip. Under the joint effect of DEP and fluid drag, white blood cells and circulating tumor cells are separated because of different dielectric properties. First, the mathematical models of device geometry, single cell, DEP force, electric field, and flow field are established to simulate the cell motion. Based on the simulation model, important boundary parameters are discussed to optimize the cell sorting ability of the device. A proper matching relationship between voltage and flow rate is then provided. The inlet and outlet conditions are also investigated to control the particle motion in the flow field. The significance of this study is to verify the cell separating ability of the microfluidic chip, and to provide a logistic design for the separation of rare diseased cells.
摘要
这项数值研究提出了一种基于微流控芯片的介电泳细胞分选技术. 在介电泳力和流体阻力的共同作用下, 白细胞和循环肿瘤细胞因介电特性不同而分离. 本文首先针对器件几何、 单细胞、 介电泳力、 电场和流场建立了数学模型, 模拟了细胞运动. 进而基于仿真模型, 对重要边界参数进行了讨论, 以优化该装置的细胞分选能力, 并同时提供了边界电压和流体流速之间的适当匹配关系. 此外, 还探究了流场出入口条件, 以控制其中的粒子运动. 本研究的意义在于验证了该微流控芯片的细胞分选能力, 为罕见病变细胞的分离提供了逻辑设计.
Similar content being viewed by others
References
WARKIANI M E, KHOO B L, WU L, et al. Ultra-fast, label-free isolation of circulating tumor cells from blood using spiral microfluidics [J]. Nature Protocols, 2016, 11(1): 134–148.
GUPTA G P, MASSAGUÉ J. Cancer metastasis: Building a framework [J]. Cell, 2006, 127(4): 679–695.
ABDULLA A, LIU W J, GHOLAMIPOUR-SHIRAZI A, et al. High-throughput isolation of circulating tumor cells using cascaded inertial focusing microfluidic channel [J]. Analytical Chemistry, 2018, 90(7): 4397–4405.
YAN B, FU S J, CHANG Y Y, et al. Mutational analysis of OCT4+ and OCT4− circulating tumour cells by single cell whole exome sequencing in stage I non-small cell lung cancer patients [J]. Journal of Shanghai Jiao Tong University (Science), 2021, 26(1): 40–46.
MICALIZZI D S, MAHESWARAN S, HABER D A. A conduit to metastasis: Circulating tumor cell biology [J]. Genes & Development, 2017, 31(18): 1827–1840.
KIM H J, JANG W K, KIM B H, et al. Advancing liquid front shape control in capillary filling of microchannel via arrangement of microposts for microfluidic biomedical Sensors [J]. International Journal of Precision Engineering and Manufacturing, 2016, 17(1): 59–63.
SARKAR A, HOU H W, MAHAN A E, et al. Multiplexed affinity-based separation of proteins and cells using inertial microfluidics [J]. Scientific Reports, 2016, 6: 23589.
MARISCAL J, ALONSO-NOCELO M, MUINELOROMAY L, et al. Molecular profiling of circulating tumour cells identifies Notch1 as a principal regulator in advanced non-small cell lung cancer [J]. Scientific Reports, 2016, 6: 37820.
CHEN Q, YAO L, BURNER D, et al. Epithelial membrane protein 2: A novel biomarker for circulating tumor cell recovery in breast cancer [J]. Clinical and Translational Oncology, 2019, 21(4): 433–442.
GOU Y X, ZHANG S, SUN C K, et al. Sheathless inertial focusing chip combining a spiral channel with periodic expansion structures for efficient and stable particle sorting [J]. Analytical Chemistry, 2020, 92(2): 1833–1841.
JOHNSTON I D, MCDONNELL M B, TAN C K L, et al. Dean flow focusing and separation of small microspheres within a narrow size range [J]. Microfluidics and Nanofluidics, 2014, 17(3): 509–518.
MALEKMOHAMMADI M, AKHLAGHI E A, SOLTANI J, et al. Escape velocity sorting in optical tweezers system using a home-made piezo mirror [J]. Journal of Optics, 2020, 22(5): 055301.
TEWARI KUMAR P, DECROP D, SAFDAR S, et al. Digital microfluidics for single bacteria capture and selective retrieval using optical tweezers [J]. Micromachines, 2020, 11(3): 308.
ANTFOLK M, MAGNUSSON C, AUGUSTSSON P, et al. Acoustofluidic, label-free separation and simultaneous concentration of rare tumor cells from white blood cells [J]. Analytical Chemistry, 2015, 87(18): 9322–9328.
GU Y Y, CHEN C Y, MAO Z M, et al. Acoustofluidic centrifuge for nanoparticle enrichment and separation [J]. Science Advances, 2021, 7(1): eabc0467.
SHI J Y, LI S Y, ZHANG X F. The acoustic radiation force on a multi-layered polymer capsule placed in a fluid-filled tube [J]. Ultrasonics, 2021, 113: 106365.
PIACENTINI N, MERNIER G, TORNAY R, et al. Separation of platelets from other blood cells in continuous-flow by dielectrophoresis field-flow-fractionation [J]. Biomicrofluidics, 2011, 5(3): 34122–34128.
VAN DEN DRIESCHE S, RAO V, PUCHBERGERENENGL D, et al. Continuous cell from cell separation by traveling wave dielectrophoresis [J]. Sensors and Actuators B: Chemical, 2012, 170: 207–214.
RASHED M Z, WILLIAMS S J. Advances and applications of isomotive dielectrophoresis for cell analysis [J]. Analytical and Bioanalytical Chemistry, 2020, 412(16): 3813–3833.
ABT V, GRINGEL F, HAN A, et al. Separation, characterization, and handling of microalgae by dielectrophoresis [J]. Microorganisms, 2020, 8(4): 540.
LIN X G, YAO J, DONG H, et al. Effective cell and particle sorting and separation in screen-printed continuous-flow microfluidic devices with 3D sidewall electrodes [J]. Industrial & Engineering Chemistry Research, 2016, 55(51): 13085–13093.
ALAZZAM A, MATHEW B, ALHAMMADI F. Novel microfluidic device for the continuous separation of cancer cells using dielectrophoresis [J]. Journal of Separation Science, 2017, 40(5): 1193–1200.
MILOH T, NAGLER J. Travelling-wave dipolophoresis: Levitation and electrorotation of Janus nanoparticles [J]. Micromachines, 2021, 12(2): 114.
SIM K, SHI L L, HE G L, et al. Mechanically flexible microfluidics for microparticle dispensing based on traveling wave dielectrophoresis [J]. Journal of Micromechanics and Microengineering, 2020, 30(2): 024001.
EGGER M, DONATH E. Electrorotation measurements of diamide-induced platelet activation changes [J]. Biophysical Journal, 1995, 68(1): 364–372.
GARCIA-SANCHEZ P, REN Y K, ARCENEGUI J J, et al. Alternating Current electrokinetic properties of gold-coated microspheres [J]. Langmuir, 2012, 28(39): 13861–13870.
TRAINITO C I, BAYART E, SUBRA F, et al. The electrorotation as a tool to monitor the dielectric properties of spheroid during the permeabilization [J]. The Journal of Membrane Biology, 2016, 249(5): 593–600.
HUANG L, ZHAO P, LIANG F, et al. Single-cell 3D electro-rotation [J]. Methods in Cell Biology, 2018, 148: 97–116.
KIRBY B. Micro- and nanoscale fluid mechanics [M]. Cambridge: Cambridge University Press, 2009.
GASCOYNE P R C, VYKOUKAL J. Particle separation by dielectrophoresis [J]. Electrophoresis, 2002, 23(13): 1973–1983.
ALI H, PARK C W. Numerical study on the complete blood cell sorting using particle tracing and dielectrophoresis in a microfluidic device [J]. Korea-Australia Rheology Journal, 2016, 28(4): 327–339.
HENSLEE E A, SANO M B, ROJAS A D, et al. Selective concentration of human cancer cells using contactless dielectrophoresis [J]. Electrophoresis, 2011, 32(18): 2523–2529.
LANNIN T, SU W W, GRUBER C, et al. Automated electrorotation shows electrokinetic separation of pancreatic cancer cells is robust to acquired chemotherapy resistance, serum starvation, and EMT [J]. Biomicrofluidics, 2016, 10(6): 064109.
FUHR G, GLASSER H, MÜLLER T, et al. Cell manipulation and cultivation under a.c. electric field influence in highly conductive culture media [J]. Biochimica et Biophysica Acta, 1994, 1201(3): 353–360.
COTTET J, FABREGUE O, BERGER C, et al. MyDEP: A new computational tool for dielectric modeling of particles and cells [J]. Biophysical Journal, 2019, 116(1): 12–18.
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation item: the Base for Interdisciplinary Innovative Talent Training Project
Rights and permissions
About this article
Cite this article
Wang, Y., Ding, X. & Zhang, Z. Numerical Study on Separation of Circulating Tumor Cell Using Dielectrophoresis in a Four-Electrode Microfluidic Device. J. Shanghai Jiaotong Univ. (Sci.) 28, 391–400 (2023). https://doi.org/10.1007/s12204-022-2459-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12204-022-2459-9