Abstract
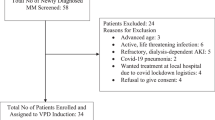
The aim of the present study was to evaluate the effectiveness of bortezomib combined with epirubicin, dexamethasone, and thalidomide (BADT) for the treatment of multiple myeloma (MM). The BADT regimen consisted of a maximum of eight 4-week cycles of: intravenous bortezomib (1.0 mg/m2) and intravenous epirubicin (12 mg/m2) on days 1, 4, 8, and 11; dexamethasone (20 mg) on days 1, 2, 4, 5, 8, 9, 11, and 12; and oral thalidomide (100 mg/m2) on days 1–28. Twelve patients with MM were included in the study, of whom four had not been previously treated and eight had been previously treated with at least one cycle of a systemic combined regimen. All the patients completed at least two cycles of treatment, with an average of five cycles; the complete response (CR) rate was 83.3% (10/12) and stabilization of disease was 16.7% (2/12). The average number of cycles required to achieve CR was 1.9 (range 1–6). In three patients, mobilization of peripheral blood stem cells allowed a sufficient quantity of CD34+ cells to be harvested for future autotransplantation. The main adverse reactions included peripheral neuropathy (4/12), thrombocytopenia (3/12), electrocardiographic abnormalities (4/12), neutropenia (5/12), and liver function impairment (4/12), primarily grade I–II. Infection occurred in four patients with neutropenia, including one patient who developed sepsis. The estimated 1-year overall survival rate was 91.7 ± 8.0%, and the estimated 1-year disease-free survival was 75.0 ± 12.5%. BADT is a highly effective combined regimen, with acceptable toxicity, for the treatment of multiple myeloma.
Similar content being viewed by others
References
Sharma NL, Sharma VC, Mahajan VK, Shanker V, Ranjan N, Gupta M. Thalidomide: an experience in therapeutic outcome and adverse reactions. J Dermatolog Treat. 2007;21:1–6.
Dimopoulos MA, Zervas K, Kouvatseas G, et al. Thalidomide and dexamethasone combination for refractory multiple myeloma. Ann Oncol. 2001;12:991–5.
Hideshima T, Richardson P, Chauhan D, et al. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 2001;61:3071–6.
Ma MH, Yang HH, Parker K, et al. The proteasome inhibitor PS-341 markedly enhances sensitivity of multiple myeloma tumor cells to chemotherapeutic agents. Clin Cancer Res. 2003;9:1136–44.
Mitsiades N, Mitsiades CS, Richardson PG, et al. The proteasome inhibitor PS-341 potentiates sensitivity of multiple myeloma cells to conventional chemotherapeutic agents: therapeutic applications. Blood. 2003;101:2377–80.
Richardson PG, Barlogie B, Berenson J, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348:2609–17.
Orlowski RZ, Nagler A, Sonneveld P, et al. Randomized phase III study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone in relapsed or refractory multiple myeloma: combination therapy improves time to progression. J Clin Oncol. 2007;25:3892–901.
Richardson PG, Sonneveld P, Schuster M, et al. Extended follow-up of a phase 3 trial in relapsed multiple myeloma: final time-to-event results of the APEX trial. Blood. 2007;110:3557–60.
Rosiñol L, Oriol A, Mateos MV, et al. Phase II PETHEMA trial of alternating bortezomib and dexamethasone as induction regimen before autologous stem-cell transplantation in younger patients with multiple myeloma: efficacy and clinical implications of tumor response kinetics. J Clin Oncol. 2007;25:4452–8.
Freimann H, Calderoni A, Cornu P, Olie R. Daily practice use of Bortezomib in relapsed/refractory multiple myeloma. Safety/efficacy results of a compassionate use program in Switzerland. Swiss Med Wkly. 2007;137:317–22.
Biehn SE, Moore DT, Voorhees PM, et al. Extended follow-up of outcome measures in multiple myeloma patients treated on a phase I study with bortezomib and pegylated liposomal doxorubicin. Ann Hematol. 2007;86:211–6.
Wang M, Giralt S, Delasalle K, Handy B, Alexanian R. Bortezomib in combination with thalidomide-dexamethasone for previously untreated multiple myeloma. Hematology. 2007;12:235–9.
Kropff M, Bisping G, Schuck E, et al. Bortezomib in combination with intermediate-dose dexamethasone and continuous low-dose oral cyclophosphamide for relapsed multiple myeloma. Br J Haematol. 2007;138:330–7.
Barlogie B, Desikan R, Eddlemon P, et al. Extended survival in advanced and refractory multiple myeloma after single-agent thalidomide: identification of prognostic factors in a phase 2 study of 169 patients. Blood. 2001;98:492–4.
Badros A, Goloubeva O, Fenton R, et al. Phase I trial of first-line bortezomib/thalidomide plus chemotherapy for induction and stem cell mobilization in patients with multiple myeloma. Clin Lymphoma Myeloma. 2006;7:210–6.
Palumbo A, Ambrosini MT, Benevolo G, et al. Bortezomib, melphalan, prednisone, and thalidomide for relapsed multiple myeloma. Blood. 2007;109:2767–72.
Oakervee HE, Popat R, Curry N, et al. PAD combination therapy (PS-341/bortezomib, doxorubicin and dexamethasone) for previously untreated patients with multiple myeloma. Br J Haematol. 2005;129:755–62.
Ciolli S, Leoni F, Gigli F, Rigacci L, Bosi A. Low dose Velcade, thalidomide and dexamethasone (LD-VTD): an effective regimen for relapsed and refractory multiple myeloma patients. Leuk Lymphoma. 2006;47:171–3.
Badros A, Goloubeva O, Dalal JS, et al. Neurotoxicity of bortezomib therapy in multiple myeloma: a single-center experience and review of the literature. Cancer. 2007;110:1042–9.
Jagannath S, Durie BG, Wolf J, et al. Bortezomib therapy alone and in combination with dexamethasone for previously untreated symptomatic multiple myeloma. Br J Haematol. 2005;129:776–83.
Tong Y, Qian J, Li Y, Meng H, Jin J. The high incidence of varicella herpes zoster with the use of bortezomib in 10 patients. Am J Hematol. 2007;82:403–4.
Enrico O, Gabriele B, Nadia C, et al. Unexpected cardiotoxicity in haematological bortezomib treated patients. Br J Haematol. 2007;138:396–7.
Acknowledgments
This work was supported by the Shanghai science and technology grant: 064119511, 07ZR14146.
Conflict of interest disclosure
The authors declare no competing financial interests.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Lü, S., Wang, J., Xu, X. et al. Bortezomib in combination with epirubicin, dexamethasone and thalidomide is a highly effective regimen in the treatment of multiple myeloma: a single-center experience. Int J Hematol 89, 34–38 (2009). https://doi.org/10.1007/s12185-008-0218-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-008-0218-9