Abstract
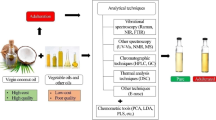
Coconut oil is the main edible oil used in South and Southeast Asian countries. Different types of coconut oils are available in the market including virgin coconut oil (VCO), copra coconut oil (CCO), coconut oil extracted by desiccated coconut (DC oil), and refined bleached and deodorized coconut oil based on the manufacturing process. Due to recently identified medical and cosmetic benefits, a huge market has opened for VCO. On top of that, vendors tend to mislead consumers by marketing DC oil instead of VCO for unscriptural financial gain. A reliable accurate method was developed to differentiate VCO from other coconut oils based on their process-based markers. A headspace solid-phase microextraction sampling method combined with gas chromatography was used for the isolation and separation of volatiles, respectively. Mass chromatography was used for the analysis of different constituents in coconut oils. A set of unique chemical compounds was identified for each coconut oil type such as 2-pentanone for VCO extracted from the dry method, hexanal for CCO, and acetic acid for VCO extracted from wet method. It was able to identify four compounds, δ-caprolactone, oxime-methoxy-phenyl, δ-octalactonone, and δ-decalactone which were common to oil coconut oil types. Since these unique compounds are only available in particular oil types, it enables them to be used as marker or tracer compounds to confirm their authenticity.


Similar content being viewed by others
Data availability
Additional data will be provided with reasonable request.
References
Bansal A, Kirschnera M, Zub L, Caib D, Zhang L (2019) Coconut oil decreases expression of amyloid precursor protein (APP) and secretion of amyloid peptides through inhibition of ADP-ribosylation factor 1 (ARF1). Brain Res 1704:78–84
Cecchi L, Migliorini M, Giambanelli E, Cane A, Mulinacci N, Zanoni B (2021) Volatile profile of two-phase olive pomace (alperujo) by HS-SPME GC−MS as a key to defining volatile markers of sensory defects caused by biological phenomena in virgin olive oil. J Agric Food Chem 69:5155–5166
Chang M, Zhao P, Zhang T, Wang Y, Guo X, Jin Q, Wang X (2020) Characteristic volatile fingerprints and profiles determination in different grade of coconut oil by HS-GC-IMS and HS-SPME-GC-MS. Int J Food Sci Technol 55(12):3670–3679. https://doi.org/10.1111/ijfs.14664
CODEX 210 (2019) Codex standard for named vegetable oils, Codex standard for fats and oils from vegetable sources
Dayrit FM, Dimzon IKD, Valde MF, Santos JER, Garrovillas MJM, Villarino BJ (2011) Quality characteristics of virgin coconut oil: comparisons with refined coconut oil. Pure Appl Chem 83(9):1789–1799
DebMandal M, Mandal S (2011) Coconut (Cocos nucifera L.: Arecaceae): in health promotion and disease prevention. Asian Pac J Trop Med 4(3):241–247
Dimzon I K D, Tantengco G B, Oquendo N A, Dayrit FM (2021) Profile of volatile organic compounds (VOCs) from cold processed and heat-treated virgin coconut oil (VCO) samples. Proceedings 70(1):70–85. https://doi.org/10.3390/foods_2020-07723
ISO 3961(2013E) Animal and vegetable fats and oils - determination of iodine value. International Standard Organization
ISO 3657(2013E) Animal and vegetable fats and oils - determination of saponification value. International Standard Organization
ISO 12966–2(2017E) Animal and vegetable fats and oils — gas chromatography of fatty acid methyl esters — part 2: preparation of methyl esters of fatty acids. International Standard Organization
Lee PR, Boo CX, Liu SQ (2013) Fermentation of coconut water by probiotic strains Lactobacillus acidophilus L10 and Lactobacillus casei L26. Ann Microbiol 63:1441–1450
Marina AM, Che Man YB, Nazimah SAH, Amin I (2009a) Chemical properties of virgin coconut oil. J Am Oil Chem Soc 86:301–307
Marina AM, Che Man YB, Nazimah ASH, Amin I (2009b) Antioxidant capacity and phenolic acids of virgin coconut oil. Int J Food Sci Nutr 60(S2):114–123
Mulyadi AF, Schreiner M, Dewi IA (2021) Phenolic and volatile compounds, antioxidant activity, and sensory properties of virgin coconut oil: occurrence and their relationship with quality. AIP Conf Proc 070020:1–4. https://doi.org/10.1063/1.5062818
Nawar WW (1984) Chemical changes in lipid produced by thermal processing. J Chem Educ 61:299–302
Perestrelo R, Silva C, Silva P, Câmara JS (2017) Global volatile profile of virgin olive oils flavoured by aromatic/medicinal plants. Food Chem 227:111–121
Pethiyagoda U (1980) Handbook on coconut cultivation coconut research (1st ed.). Coconut Research Institute of Sri Lanka, pp 1–4
Rohman A, Irnawati, Erwanto Y, Lukitaningsih E, Rafi M, Fadzilah N A, Windarsih A, Sulaiman A, Zakaria Z (2019) Virgin coconut oil: extraction, physicochemical properties, biological activities and its authentication analysis. Food Rev Int 37(1): 46–66. https://www.tandfonline.com/doi/abs/10.1080/87559129.2019.1687515
Romero I, García-González DL, Aparicio-Ruiz R, Morales MT (2015) Validation of SPME–GC MS method for the analysis of virgin olive oil volatiles responsible for sensory defects. Talanta 134:394–401
Saittagaroon S, Kawakishi S, Namiki M (1984) Aroma constituents of roasted coconut. Agric Biol Chem 48(9):2301–2307
Santos JER, Villarino BJ, Zosa AR, Dayrit FM (2011) Analysis of volatile organic compounds in virgin coconut oil and their sensory attributes. Philipp J Sci 140(2):161–171
Satheesh N, Prasad NBL (2014) Production of virgin coconut oil by induced fermentation with Lactobacillus plantarum NDRI strain 184. Croat J Food Sci Tech 9(1–2):37–42
Satheeshan KN, Seema BR, Manjusha AVM (2019) Quality analysis of virgin coconut oil processed through different methods. J Pharmacogn Phytochem 8(3):2119–2123
Seneviratne KN, Hapuarachchi CD, Ekanayake S (2009) Comparison of the phenolic-dependent antioxidant properties of coconut oil extracted under cold and hot conditions. Food Chem 114:1444–1449
SLS 32:2017. Sri Lanka standard specification for coconut oil (3rd rev). SLS standard, Sri Lanka: Sri Lanka Standard Institute
Srivastava Y, Semwal AD, Majumdar A (2016) Quantitative and qualitative analysis of bioactive components present in virgin coconut oil. Cogent Food Agric 2:1164929. https://doi.org/10.1080/23311932.2016.1164929
Sun Y, Dou X, Yue X, Yu L, Zhang L, Li L, Li P (2020) Optimization of headspace SPME GC × GC-TOF/MS analysis of volatile organic compounds in edible oils by central composite design for adulteration detection of edible oil. Food Anal Methods 13:1328–1336. https://doi.org/10.1007/s12161-020-01741-3
Suzuki D, Sato Y, Kamasaka H T, Tamura H (2020) Oiling-out effect improves the efficiency of extracting aroma compounds from edible oil. Sci Food https://doi.org/10.1038/s41538-020-00079-8
U.S. United States Department of Agriculture Foreign Agricultural Service (2022) Oilseeds: world markets and trade, Retrieved September 30, 2022 from the US Department of Agriculture Foreign Agricultural Service page: https://www.fas.usda.gov/data/oilseeds-world-markets-and-trade. Accessed 30 Sept 2022
Acknowledgements
Authors acknowledge treasury grants of Sri Lanka (TG 19/189, 2019) for the financial assistance to conduct research work.
Funding
The work was supported by funding Treasury Grant of Sri Lanka under the grant number TG 19/189.
Author information
Authors and Affiliations
Contributions
HGTHJ: writing the article and investigation. HDW: technical support. HPPSS: conceptualization, supervision, and review the article. KRRM: supervision and review the article.
Corresponding author
Ethics declarations
Authors declare that the findings presented in the article are purely generated in the Industrial Technology Institute Sri Lanka. These data has not published in elsewhere.
Ethics Approval
This article does not contain any studies with human participants or animals performed by any of authors.
Competing Interests
H G T H Jayatunga declares there is no financial interest. Only some parts of this paper have been used for a patent application on the date of 2023/02/23 to the National intellectual property office of Sri Lanka. H D Weerathunga declares there is no financial interest. Only some parts of this paper have been used for a patent application on the date of 2023/02/23 to the National intellectual property office of Sri Lanka. H P P S Somasiri declares there is no financial interest. Only some parts of this paper have been used for a patent application on the date of 2023/02/23 to the National intellectual property office of Sri Lanka. K R R Mahanam declares there is no financial interest. Only some parts of this paper have been used for a patent application on the date of 2023/02/23 to the National intellectual property office of Sri Lanka.
Conflict of Interest
H. G. T. H. Jayatunga declares that there is no financial interest. Only some parts of this paper have been used for a patent application on the date of 2023/02/23 to the National Intellectual Property Office of Sri Lanka. H. D. Weerathunge declares that there is no financial interest. Only some parts of this paper have been used for a patent application on the date of 2023/02/23 to the National Intellectual Property Office of Sri Lanka. H. P. P. S. Somasiri declares that there is no financial interest. Only some parts of this paper have been used for a patent application on the date of 2023/02/23 to the National Intellectual Property Office of Sri Lanka. K. R. R. Mahanam declares that there is no financial interest. Only some parts of this paper have been used for a patent application on the date of 2023/02/23 to the National Intellectual Property Office of Sri Lanka.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jayatunga, H.G.T.H., Weerathunge, H.D., Somasiri, H.P.P.S. et al. Use of Process-Based Marker Compounds to Identify Different Coconut Oils. Food Anal. Methods 17, 96–104 (2024). https://doi.org/10.1007/s12161-023-02552-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-023-02552-y