Abstract
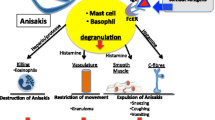
Wild-caught marine fish are potentially carrying parasites. Larvae of the nematode Anisakis simplex (herring or whale worm) occur in almost all commercially exploited fish stocks in temperate seas. The presence of A. simplex in fish and fish products is not only an economic concern but represents a significant consumer health risk. Anisakiasis, human infection with live larvae, can occur by consuming raw or undercooked fish while allergy symptoms can also be elicited by the presence of A. simplex proteins in processed seafood. The European Food Safety Authority (EFSA) has concluded in a scientific opinion that routine testing of seafood products for A. simplex is needed. In the present study, we have determined A. simplex proteins in farmed salmon intended for use in sushi and fish products from the Norwegian market by quantitative sandwich ELISA, immunostaining and mass spectrometry. Analytical methods detecting anisakid proteins at the single-digit milligram level appear to be sufficiently sensitive for the protection of allergic consumers. Only trace amounts (<10 mg/kg) were detected in a few samples showing that contamination with A. simplex is apparently not an immediate health problem given the results from this survey.





Similar content being viewed by others
References
AAITO-IFIACI Anisakis Consortium (2011) Anisakis hypersensitivity in Italy: prevalence and clinical features: a multicenter study. Allergy 66:1563–1569
Abbott M, Hayward S, Ross W, Godefroy SB, Ulberth F, Van Hengel AJ, Roberts J, Akiyama H, Popping B, Yeung JM, Wehling P, Taylor SL, Poms RE, Delahaut P (2010) Validation procedures for quantitative food allergen ELISA methods: community guidance and best practices. J AOAC Int 93:442–450
Adams AM, Ton MN, Wekell MM, MacKenzie AP, Dong FM (2005) Survival of Anisakis simplex in arrowtooth flounder (Atheresthes stomia) during frozen storage. J Food Prot 68:1441–1446
Angot V, Brasseur P (1993) European farmed Atlantic salmon (Salmo salar L.) are safe from anisakid larvae. Aquaculture 118:339–344
Arilla MC, Ibarrola I, Martínez A, Monteseirín J, Conde J, Asturias JA (2008) An antibody-based ELISA for quantification of Ani s 1; a major allergen from Anisakis simplex. Parasitol 135:735–740
Armentia A, Martin-Gil FJ, Pascual C, Martín-Esteban M, Callejo A, Martínez C (2006) Anisakis simplex allergy after eating chicken meat. J Investig Allergol Clin Immunol 16:258–263
Audicana MT, Kennedy MW (2008) Anisakis simplex: from obscure infectious worm to inducer of immune hypersensitivity. Clin Microbiol Rev 21:360–369
Audicana MT, Ansotegui IJ, de Corres LF, Kennedy MW (2002) Anisakis simplex: dangerous–dead and alive? Trends Parasitol 18:20–25
Bristow GA, Berland B (1991) A report on metazoan parasites of wild marine salmon (Salmo salar L.) from the west-coast of Norway with comments on their interaction with farmed salmon. Aquaculture 98:311–318
Caballero ML, Moneo I (2004) Several allergens from Anisakis simplex are highly resistant to heat and pepsin treatments. Parasitol Res 93:248–251
Codex Alimentarius Commission (2005) Report of the 27th session of the codex committee on fish and fishery products. www.codexalimentarius.org/input/download/report/633/al28_18e.pdf. Acessed 4–9 July 2005
Daschner A, Pascual C-Y (2005) Anisakis simplex: sensitization and clinical allergy. Curr Opin Allergy Clin Immunol 5:281–285
Daschner A, Alonso-Gómez A, Cabañas R, Suarez-de-Parga JM, López-Serrano MC (2000) Gastroallergic anisakiasis: borderline between food allergy and parasitic disease-clinical and allergologic evaluation of 20 patients with confirmed acute parasitism by Anisakis simplex. J Allergy Clin Immunol 105:176–181
de Corres FL, Audicana M, Del Pozo MD, Muñoz D, Fernández E, Navarro JA, Garcia M, Diez J (1996) Anisakis simplex induces not only anisakiasis: report on 28 cases of allergy caused by this nematode. J Investig Allergol Clin Immunol 6:315–319
Deardorff TL, Kent ML (1989) Prevalence of larval Anisakis simplex in pen-reared and wild-caught salmon (Salmonidae) from Puget Sound, Washington. J Wildl Dis 25:416–419
EFSA Panel on Biological Hazards (BIOHAZ) (2010) Scientific opinion on risk assessment of parasites in fishery products. EFSA J 8:1543
Espiñeira M, Herrero B, Vieites JM, Santaclara FJ (2010) Detection and identification of anisakids in seafood by fragment length polymorphism analysis and PCR–RFLP of ITS-1 region. Food Contr 21:1051–1060
European Commission Regulation No 1276/2011 (2011) Amending annex III to regulation (EC) No 853/2004 of the European parliament and of the council as regards the treatment to kill viable parasites in fishery products for human consumption. http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2011:327:0039:0041:EN:PDF. Acessed 8 Dec 2011
European Commission Regulation No 853/2004 (2004) Laying down specific hygiene rules for the hygiene of foodstuffs. http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2004:139:0055:0205:EN:PDF. Acessed 29 Apr 2004
Fæste CK, Jonscher KR, Dooper MMBW, Egge-Jacobsen W, Moen A, Daschner A, Egaas E, Christians U (2014) Characterisation of potential novel allergens in the fish parasite Anisakis simplex. EuPa Open Proteom 4:140–155. doi:10.1016/j.euprot.2014.06.006
Food and Drug Administration (FDA) (2011) Fish and fishery products hazards and controls guidance. http://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/Seafood/ucm2018426.htm. Acessed Apr 2011
Food and Drug Administration (FDA) (2012) Bad bug book: handbook of foodborne pathogenic microorganisms and natural toxins. http://www.fda.gov/downloads/Food/FoodborneIllnessContaminants/UCM297627.pdf
Herrero B, Vieites JM, Espiñeira M (2011) Detection of anisakids in fish and seafood products by real-time PCR. Food Control 22:933–939
Karl H, Baumann F, Ostermeyer U, Kuhn T, Klimpel S (2011) Anisakis simplex (s.s.) larvae in wild Alaska salmon: no indication of post-mortem migration from viscera into flesh. Dis Aquat Org 94:201–209
Klimpel S, Palm HW (2011) Anisakid nematode (Ascaridoidea) life cycles and distribution: increasing zoonotic potential in the time of climate change? In: Melhorn H (ed) Progress in parasitology, 1st edn. Springer-Verlag, Berlin, pp 201–222
Levsen A, Lunestad BT (2010) Anisakis simplex third stage larvae in Norwegian spring spawning herring (Clupea harengus L.), with emphasis on larval distribution in the flesh. Vet Parasitol 171:247–253
Levsen A, Fæste CK, Lin AH, Van Do T, Florvåg E, Egaas E (2013) Consumer health risk posed by Anisakis simplex in Northern Europe—an update. Trop Med Int Health 18:49
Lin AH, Florvaag E, Van Do T, Johansson SG, Levsen A, Vaali K (2012) IgE sensitization to the fish parasite Anisakis simplex in a Norwegian population: a pilot study. Scand J Immunol 75:431–435
Lopez I, Pardo MA (2010) Evaluation of a real-time polymerase chain reaction (PCR) assay for detection of Anisakis simplex parasite as a food-borne allergen source in seafood products. J Agri Food Chem 58:1469–1677
Lunestad BT (2003) Absence of nematodes in farmed Atlantic salmon (Salmo salar L.) in Norway. J Food Protect 1:122–124
Marty GD (2008) Anisakid larva in the viscera of a farmed Atlantic salmon (Salmo salar). Aquaculture 279:209–210
Mo TA, Gahr A, Hansen H, Hoel E, Oaland Ø, Poppe TT (2014) Presence of Anisakis simplex (Rudolphi, 1809 det. Krabbe, 1878) and Hysterothylacium aduncum (Rudolphi, 1802) (Nematoda; Anisakidae) in runts of farmed Atlantic salmon, Salmo salar L. J Fish Dis 37:135–140
Moneo I, Caballero ML, González-Muñoz M, Rodríguez-Mahillo AI, Rodríguez-Perez R, Silva A (2005) Isolation of a heat-resistant allergen from the fish parasite Anisakis simplex. Parasitol Res 96:285–289
Mossali C, Palermo S, Capra E, Piccolo G, Botti S, Bandi C, D’Amelio S, Giuffra E (2010) Sensitive detection and quantification of anisakid parasite residues in food products. Foodborne Pathog Dis 7:391–397
Petrie A, Wootten R, Bruno D, MacKenzie K, Bron J (2007) A survey of Anisakis and Pseudoterranova in Scottish fisheries and the efficacy of current detection methods. Food Standards Agency Scotland Project S14008
Pravettoni V, Primavesi L, Piantanida M (2012) Anisakis simplex: current knowledge. Eur Ann Allergy Clin Immunol 44:150–156
Rodríguez-Mahillo AI, González-Muñoz M, de las Heras C, Tejada M, Moneo I (2010) Quantification of Anisakis simplex allergens in fresh, long-term frozen, and cooked fish muscle. Foodborne Pathog Dis 7:967–973
Sakanari JA, McKerrow JH (1989) Anisakiasis. Clin Microbiol Rev 2:278–284
Sastre J, Lluch-Bernal M, Quirce S, Arrieta I, Lahoz C, Del Amo A, Fernández-Caldas E, Marañón F (2000) A double-blind, placebo-controlled oral challenge study with lyophilized larvae and antigen of the fish parasite, Anisakis simplex. Allergy 55:560–564
Sivertsen AH, Heia K, Hindberg K, Godtliebsen F (2012) Automatic nematode detection in cod fillets (Gadus morhua L.) by hyperspectral imaging. J Food Eng 111:675–681
Smith JW, Wootten R (1975) Experimental studies on the migration of Anisakis sp. larvae (Nematoda: ascaridida) into the flesh of herring, Clupea harengus L. Int J Parasitol 5:133–136
Taylor SL, Baumert JL, Kruizinga AG, Remington BC, Crevel RW, Brooke-Taylor S, Allen KJ, Allergen Bureau of Australia & New Zealand, Houben G (2014) Establishment of reference doses for residues of allergenic foods: report of the VITAL expert panel. Food Chem Toxicol 63:9–17
Torres-Frenzel P, Torres P (2014) Anisakid parasites in commercial hake ceviche in Southern Chile. J Food Protect 7:1037–1240
Umehara A, Kawakami Y, Araki J, Uchida A (2008) Multiplex PCR for the identification of Anisakis simplex sensu stricto, Anisakis pegreffii and other anisakid nematodes. Parasitol Int 57:49–53
Vidaček S, De Las HC, Solas MT, García ML, Mendizábal A, Tejada M (2011) Viability and antigenicity of anisakis simplex after conventional and microwave heating at fixed temperatures. Food Protect 74:2119–2126
Weir E (2005) Sushi, nematodes and allergies. Can Med Assoc J 172:329
Werner MT, Fæste CK, Levsen A, Egaas E (2011) A quantitative sandwich ELISA for the detection of Anisakis simplex protein in seafood. Eur Food Res Technol 232:157–166
Wootten R, Yoon G-H, Bron J (2010) A survey of anisakid nematodes in Scottish wild Atlantic salmon. Food standards agency Scotland project S14008
Acknowledgments
The authors would like to thank Dr. Arne Levsen from the National Institute of Nutrition and Seafood in Bergen, Norway, for the sourcing and characterisation of Anisakis simplex third-stage larvae. We greatly appreciate Marianne Werner from the Norwegian Veterinary Institute for preparing the protein extracts and Dr. Jan Haug Anonsen from the University of Oslo for supporting the mass spectrometry analysis. We are also very grateful to Dr. Alvaro Daschner from the Instituto de Investigacion Sanitaria at the Hospital Universitario de La Princesa in Madrid, Spain, for providing serum from a patient with allergy to A. simplex. Special thanks also to Sylvi Anita Olsen from the Norwegian Food Safety Authority for good cooperation and coordination of the sushi study. The Orkla Foundation, Norway, has funded this project.
Conflict of Interest
Christiane Kruse Fæste declares that she has no conflict of interest. Christin Plassen declares that she has no conflict of interest. Kjersti Eriksen Løvberg declares that she has no conflict of interest. Anders Moen declares that he has no conflict of interest. Eliann Egaas declares that she has no conflict of interest. This article does not contain any studies with human or animal subjects.
Ethics Requirements
The polyclonal antibodies were produced in a previous project (for details, see Werner et al. 2011) in rabbits in accordance with the Norwegian guidelines for the care and use of laboratory animals.
Human serum was obtained and used by informed consent in a previous study (for details, see Fæste et al. 2014) in accordance with the ethical standards of the Hospital Universitario de La Princesa in Madrid, Spain.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fæste, C.K., Plassen, C., Løvberg, K.E. et al. Detection of Proteins from the Fish Parasite Anisakis simplex in Norwegian Farmed Salmon and Processed Fish Products. Food Anal. Methods 8, 1390–1402 (2015). https://doi.org/10.1007/s12161-014-0003-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-014-0003-8