Abstract
A series of batch assays have been conducted to investigate the optimal factors that can be adopted to improve the anaerobic digestion (AD) performance of Phragmites australis and increase biogas production. The assays were carried out using 125 mL microcosm reactors with a working volume of 80 mL and incubated at mesophilic conditions (37 ± 1ºC). The effect of particle size (10, 5, 2, and < 1 mm) and alkaline pre-treatment of P. australis using various concentrations of sodium hydroxide (0.5, 1, 2, and 4%) on biogas production was examined. Furthermore, the best pre-treatment incubation time (12, 24, 48, 72, 96, and 120 h) and the optimal inoculum to substrate ratio (ISR: 4:1, 2:1, 1:1, 1:2 and 1:4) were also assessed. The results revealed that the highest biogas production from P. australis was achieved at particle size < 1 mm (27.97 ± 0.07 and 16.67 ± 0.09 mL/g VS added, for pre-treated and untreated P. australis respectively); 2% and 4% NaOH concentration for pre-treatment (70.01 ± 3.75 and 76.14 ± 2.62 mL/g VS added, respectively); pre-treatment incubation time of 72, 96, and 120 h (71.18 ± 1.79, 72.46 ± 1.08, and 73.78 ± 1.87 mL/g VS added, respectively); and ISR of 1:2 for pre-treated P. australis (78.21 ± 0.36 mL/g VS added) and ISR 1:4 for untreated P. australis (28.93 ± 1.55 mL/g VS added). Determining optimal parameters in this work would guide further development of process configurations, such as continuous AD systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the past few decades, overconsumption of fossil fuels has led to increased greenhouse gas emissions, aggravating global warming, and transforming it into a paramount environmental concern worldwide. Hence, adopting renewable energy sources, such as solar, wind, and biofuel, has become crucial as an alternative to fossil fuels [1]. Consequently, biogas, one of the biofuel types, has received increasing interest in recent years [2]. For example, in the European Union, biogas production reached the equivalent of 10.9 million tonnes of oil in 2010 [3]. Furthermore, according to the International Energy Agency [4], in 2018, the consumption of 36 million tonnes of fossil fuel was displaced by using biogas. Biogas is a mixture of gases (50–75% methane, 25–50% carbon dioxide, and 0–10% other gases) that is produced through the anaerobic digestion (AD) of organic substrates [5, 6]. Biogas resulting from AD is usually used in producing electricity and heating or as fuel for transport after an upgrade to biomethane. Furthermore, the waste from the digestion of organic matter can be used as valuable fertilizer [7].
Energy crops have been widely used in AD plants as feedstocks for biogas production [8]. However, the competition of these crops with food and feed production on agricultural lands is the crucial obstacle that affected their position as a significant supplier of biomass for AD [9]. This led to the development of the trend towards the use of alternative substrates such as municipal organic waste, industrial food waste, animal manure, agricultural waste, perennial grasses, and wetland plants [10].
P. australis is one of the perennial grasses characterised by high productivity ranging from 3 up to 30 t/ha/y [11] and does not compete for arable lands, so it is probably to be one of the promising feedstocks in the field of biogas production [12]. P. australis is a tall grass; its length often ranges from 1 to 3 m and may reach 10 m in the tropics [13]. It is a widespread plant worldwide; the total area covered by P. australis beds worldwide is about 10 million hectares [12]. Wetlands are the main habitats for P. australis, in which the water table is usually slightly under the soil surface to 1 m above the soil surface [14].
In general, there are several key parameters that should be considered to examine the potential for biogas production using P. australis substrates, such as substrate particle size, substrate pre-treatment, pre-treatment incubation time, and inoculum to substrate ratio. As a lignocellulosic biomass, P. australis has a complex molecular structure causing difficulty in their digestion. Therefore, the pre-treatment step is essential to increase the biodegradability of these substrates and enhance biogas production. Physical (mechanical) pre-treatment, such as grinding, assists in reducing particle sizes and increasing the surface area, breaking down the cross-links between the cellulose, hemicellulose, and lignin components of P. australis substrate, and decreasing the crystallinity degree of cellulose, thus increasing the accessibility of hydrolysis enzymes to cellulose, and improving the digestion process and gas production. [15]. During the hydrolysis stage of the anaerobic digestion process, hydrolytic enzymes break down cellulose (carbohydrates) and other macromolecules of the P. australis substrate, such as proteins and lipids, into smaller molecules like simple sugars, amino acids, and fatty acids. These smaller molecules are then converted to organic acids and shorter volatile fatty acids at the later stage of the process and consumed by methanogens to produce biogas. Therefore, the hydrolysis step is considered a rate-limiting step as it determines the feedstock’s biodegradation rate [7].
However, mechanical treatment cannot remove the lignin, which impedes cellulose bioaccessibility [16]. Hence, other methods, such as chemical treatment, can overcome this obstacle. Alkaline treatment has preferred over other chemical treatments for treating lignocellulosic substrate due to its high ability to solubilise lignin [15], fewer inhibitors generation, and lower requirement for equipment such as complicated reactors [17]. In addition, alkaline pre-treatment can be conducted at ambient temperature and pressure [18].
In addition to the substrate particle size and alkaline pre-treatment, the inoculum-to-substrate ratio (ISR) is considered a crucial parameter that improves the performance of AD and enhances biogas production [19]. Providing optimal quantities of microbial aggregates and their nutrient requirements shortens the start-up period for biogas production and reduces the accumulation of inhibitors [20]. The appropriate selection of ISR depends on the substrate type and the digestion conditions [21]. However, when the ISR is lower than the optimum ratio, it will lead to an accumulation of VFA and inhibition of the system. In contrast, when the ISR value is higher than the optimum ratio, it will decrease the amount of nutrients required for the microorganisms [22].
To the best of the authors’ knowledge, no continuous anaerobic studies have been conducted and published on P. australis for biogas production. Therefore, the present study can be considered a basic database that provides the information on essential parameters which will guide the operation of continuous digesters to enhance biogas production. Hence, this study aims to investigate (I) the optimum particle size (10, 5, 2, and < 1 mm) of P. australis substrate that could produce the highest biogas amount during the anaerobic digestion process; (II) the effect of pre-treatment of P. australis on biogas production using various concentrations of sodium hydroxide (0.5, 1, 2, and 4%); (III) the best incubation time for NaOH treatment (12, 24, 48, 72, 96, and 120 h); and (IV) the optimal inoculum to substrate ratio (ISR = 4:1, 2:1, 1:1, 1:2 and 1:4) that increase biogas production.
Materials and Methods
The P. australis samples used in the experiments were collected from a small lake at Forrest Hills site, Lancaster, UK (54.007°N, 2.772°W). P. australis plants with a length of about 2.5 m were selected. The aboveground biomass was harvested at ~ 5–10 cm over the soil surface, and leaves and flowers were cut to get the stalks only. The P. australis stalks were packaged in sealed bags and taken to the laboratory and kept in the refrigerator at 4 °C until used in the experiments. The methanogenic inoculum was obtained from an on-farm commercial scale, mesophilic, anaerobic digester (Cockerham Green Energy Limited, UK). The inoculum was sieved through a 1-mm sieve to remove coarse materials.
Only the stalks of P. australis were used in all batch assays. In the assay that investigated the optimal particle size of P. australis for biogas production, the harvested P. australis stalks were chopped manually by scissors into four groups of particles size 10, 5, 2, and < 1 mm. While in the rest of the batch assays, the harvested P. australis stalks were ground by a knife mill into particles size < 1 mm, following which the P. australis samples were packed in sealed plastic bags and stored in the refrigerator at 4 °C until further use. Characterisation of the substrate and inoculum is presented in Table 1.
The other characteristics of the inoculum and P. australis substrate, such as carbon, hydrogen, nitrogen, sulphur, and C:N ratios, were not measured in this work. However, the inoculum used in this work was obtained from the same source as described by Gandhi et al. [23]. Thus, it is assumed to have similar carbon, hydrogen, nitrogen, sulphur, and C:N values to the inoculum used by Gandhi et al. [23]. On dry basis, the inoculum was reported to contain 34.27% carbon, 4.71% hydrogen, 4.37% nitrogen, 0.82% sulphur, and the C:N ratio was 7.84 [23]. On the other hand, the carbon, hydrogen, nitrogen, sulphur, and C:N ratio of P. australis substrate is assumed to fall within the ranges reported in the previous studies. On dry weight basis, the P. australis substrate was reported to contain 46.87–47.20% carbon, 6.38–6.77% hydrogen, 1.05–1.21% nitrogen, 0.13–0.28% sulphur, and the C:N ratio was 39.0–44.64 [24, 25].
Pre-treatment
In all the batch assays, except that which investigated the optimal NaOH concentration for P. australis treatment, the required amounts of P. australis samples were treated in a 500 mL bottle by adding 100 mL of 2% NaOH solution per 10 g of ground P. australis. Correspondingly, the untreated P. australis samples were prepared by immersing the ground P. australis in deionised water in a 500-mL bottle. One hundred milliliters of deionised water was added for every 10 g of ground P. australis. Concerning the assay that was used to determine the optimal NaOH concentration for P. australis treatment, the required amounts of P. australis were treated in a 500-mL bottle by adding 100 mL of each 0.5%, 1%, 2% and 4% NaOH solution per 10 g of ground P. australis.
After that, the bottles were covered with Parafilm on the top and incubated for 3 days at room temperature (20 ± 2 °C), except in the assay that was used to detect the optimal treatment incubation time, the bottles were incubated for 12, 24, 48, 72, 96, and 120 h. After the treatment incubation time was complete, the pre-treated and untreated P. australis substrates were sieved using a sieve (38-micron Mesh) to separate liquid and solid fractions. The solid fraction was washed with deionised water and then drained, washed, and drained until the pH reached 7. Then the treated and untreated P. australis samples were dried in the drying oven at 65 °C for 24 h.
Experiments Setup
A series of six sets of batch experiments were set up using microcosm vessels (125 mL) as reactors with working volumes of 80 mL. The inoculum, P. australis substrate and deionised water were added to reactors on mass basis (Table 2). In the first set of batch assays, the reactors were filled with inoculum and pre-treated P. australis samples (with 2% NaOH concentration for 3 days), while in the second set of batch assays, the reactors were filled with inoculum and untreated P. australis samples. The particles size of P. australis samples used in both sets of batch assays were 10, 5, 2, and < 1 mm, and the inoculum to substrate ratio (ISR) used was 4:1 based on gram volatile solid content (g VS of inoculum (wet basis)/ g VS of pre-treated and untreated P. australis (dry basis)).
The third and fourth sets of batch assays used reactors filled with pre-treated P. australis (2% NaOH concentration for 3 days) and untreated P. australis with particle sizes of < 1 mm, respectively. The inoculum and P. australis samples were added using various ISR ratios (4:1, 2:1, 1:1, 1:2, and 1:4 based on g VS content) in both sets of batch assays. The amount of inoculum in the reactors was kept constant, and the amounts of pre-treated and untreated P. australis substrate were changed to achieve the required ISRs as mentioned in the previous studies [26, 27]. In the fifth set of batch assays, the ISR of 1:2 was used. The P. australis samples with particle size < 1 mm used in this set of batch assays were pre-treated with various NaOH concentrations (4%, 2%, 1%, 0.5%, and 0.0%) for 3 days. In the sixth set of batch assays, P. australis samples with a particle size of < 1 mm that had been pre-treated with a concentration of 2% NaOH for incubation periods of 12 h, 24 h, 48 h, 72 h, 96 h, and 120 h were added to the reactors. The ISR used in this set of batch assays was 1:2 based on the g VS content of the inoculum and substrate.
Following the addition of the inoculum and substrates to the reactors, the volumes were completed by adding deionised water until reaching a working volume of 80 mL. Afterwards, the reactors were tightly sealed and purged with nitrogen gas for one minute to eliminate any remaining oxygen from the mixture and the space inside the reactors and ensure the desired anaerobic conditions were attained. Then the reactors were incubated in a water bath at mesophilic conditions (37 ± 1 ºC). All reactors were manually shaken once a day based on the procedures described in the previous studies that have investigated the effect of particle size on biogas production [28, 29].
Analytical Methods
pH values were measured using of pH meter (Mettler, Switzerland). Total solids (TS) and volatile solids (VS) of inoculum and substrates were determined based on standard methods [30]. Biogas volume was measured using biogas counters (manufactured by CJC Labs LTD, UK). The reactors were connected to the gas counters through a plastic tube. These counters contained reversible buckets and were filled with water to a level 10 mm from the lid. The bucket's volume was calibrated to hold 6 mL of the produced biogas. When produced biogas enters the counters, it will displace the water inside the buckets, causing tipping it and releasing the biogas into a small gas bag attached to the counters. Each tipping for these buckets was recorded at a data acquisition system (DAS) (manufactured by CJC Labs LTD, UK), which indicates a volume of 6 mL of the produced biogas. Biogas volume was measured at ambient temperature and corrected for standard temperature (273.15 K) and pressure (1 bar). The cumulative biogas production from reactors was expressed as mL/g VS added (VS of inoculum + VS of the substrate).
Gompertz Model
To evaluate the biogas accumulation and performance of the batch anaerobic digestion, non-linear regressions using a modified Gompertz model (Eq. 1) were performed to obtain representative simulations and predictions.
where B refers to the cumulative biogas output (mL/g VS added), Bo is the biogas generation potential (mL/g VS added), Rm is the maximal biogas generation rate (mL/g VS added/day), λ is the lag time (day), and t is the time of the experiment (days) [31].
Statistical Analyses
IBM SPSS Statistics 27 software was utilized for conducting the statistical analyses. An analysis of variance (ANOVA) was employed on the cumulative biogas production results to identify significant (p < 0.05) effects of particle size of P. australis, ISR, NaOH concentration used for pre-treating P. australis, and the incubation time for pre-treating of P. australis on gas production. a Games-Howell post hoc test (p < 0.05) was used to group the levels of variables that exhibited significant effects.
Results and Discussion
Effect of Particles Size on Biogas Production Using Pre-treated and Treated P. australis
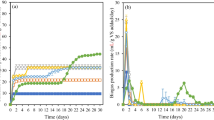
The cumulative biogas production from the digestion of pre-treated P. australis was remarkably different among the various particles size examined (Fig. 1a). The cumulative biogas produced from the digestion of pre-treated P. australis with particles size < 1 mm was significantly higher than that produced from the digestion of pre-treated P. australis with particles size 2, 5, and 10 mm. Meanwhile, it was observed that the cumulative biogas produced from treated P. australis with particles size 2 mm and 5 mm was significantly higher than that from treated P. australis with particles size 10 mm (Table 3). This can be attributed to the increased surface area and low cellulose crystallinity of the smaller particles size, which led to the greater reach of hydrolysis enzymes to the cellulose, thus increasing the hydrolysis rate of the cellulose and biogas production [15, 32].
Cumulative biogas production plots of experimental (measured) and modified-Gompertz (predicted) data from pre-treated P. australis (a) and untreated P. australis (c) at different particles size, (
 ,
,
 ) < 1 mm, (
) < 1 mm, (
 ,
,
 ) 2 mm, (
) 2 mm, (
 ,
,
 ) 5 mm, (
) 5 mm, (
 ,
,
 ) 10 mm and (
) 10 mm and (
 ,
,
 ) inoculum control; and biogas production rate from pre-treated P. australis (b) and untreated P. australis (d) at different particles size, (
) inoculum control; and biogas production rate from pre-treated P. australis (b) and untreated P. australis (d) at different particles size, (
 ) < 1 mm, (
) < 1 mm, (
 ) 2 mm, (
) 2 mm, (
 ) 5 mm, (
) 5 mm, (
 ) 10 mm and (
) 10 mm and (
 ) inoculum control, over 10 days digestion period. Data represent mean ± standard deviation
) inoculum control, over 10 days digestion period. Data represent mean ± standard deviation
Similar results were reported by Dubrovskis and Kazulis [25] as they investigated the effect of particle size on biogas production from P. australis samples harvested in the winter and summer seasons. The particle size for P. australis samples harvested in the winter period was 1, 2, 5, and 20 mm, while it was 2, 5, 7, and 20 mm for P. australis harvested in June. They found that the biogas production from P. australis with particle sizes 1 mm (from winter harvesting) and 2 mm (from summer harvesting) was higher than that produced from P. australis samples with the other particle size. Dai et al. [33] investigated the impact of particle size reduction (20, 1, 0.15, and 0.075 mm) on biogas production from anaerobic digestion of rice straw at ISR 1:2. They found that the smallest particle size showed the highest methane production of 107, 161, 182 and 197 mL/ g VS added, respectively. Sharma et al. [34] determined the biogas production from seven agricultural and forest residues with a particle size of 0.088, 0.40, 1.0, 6.0 and 30.0 mm that were anaerobically digested at ISR of 1:1. They found that the maximum methane production was achieved at smallest particle size (0.088, 0.40 mm) for all of the seven substrates. Mshandete et al. [35] investigated the anaerobic digestion of sisal fibre waste at ISR 1:2.8 and fibre sizes ranging from 2 to 100 mm. They observed an increase in methane production by 23% when the particle size was reduced to 2 mm.
With regard to biogas production from untreated P. australis, it can be seen from Fig. 1(c) that the cumulative biogas produced from untreated P. australis with particles size < 1 mm was significantly higher (p < 0.05) than that produced from untreated P. australis with particles size 2, 5, and 10 mm (Table 3), and this was consistent with what was found during the digestion of pre-treated P. australis. However, no significant differences in cumulative biogas production have appeared between the untreated P. australis with particles size 2 mm and 5 mm and between the untreated P. australis with particles size 5 mm and 10 mm (Table 3). Moreover, the digestion of untreated P. australis with particles size 2, 5 and 10 mm showed lower biogas production than inoculum reactors (controls) during the whole digestion period, and that may be due to the low breakdown of the lignin content in untreated P. australis led to limiting the bioavailability of cellulose for microbial [36].
Figure 1a shows that the estimated data of the modified Gompertz model agrees well with the experimental data. The cumulative biogas production of the model increased with the decrease in the particle size of pre-treated P. australis, as 27.47, 20.86, 18.02, and 15.03 mL/g VS added, for particle size of < 1 mm, 2 mm, 5 mm, and 10 mm, respectively. Similarly, for the untreated P. australis (Fig. 1c), the cumulative biogas outputs of the model at < 1 mm particle size were higher than that of 2, 5, and 10 mm (15.48, 11.56, 10.14, and 9.72 mL/g VS added, respectively).
On the other hand, it has appeared from Fig. 1(b and d) that the digestion of pre-treated and untreated P. australis with particle size < 1 mm showed the highest biogas production rate along the digestion period in comparison to other particle size. This indicates the faster and higher biodegradability of pre-treated and untreated P. australis at particle size < 1, making it preferable for use to improve and increase biogas production from P. australis. Besides, the high biogas production for all systems with pre-treated P. australis compared to the systems with untreated P. australis made the using of pre-treated P. australis for biogas production more feasible than using of untreated P. australis.
Effect of ISR on Biogas Production from Pre-treated P. australis (2% NaOH) and Untreated P. australis
After 32 days of digestion period, it is observed that the digestion of pre-treated P. australis at ISR of 1:2 and 1:4 presented the highest cumulative biogas production compared to that from the digestion of pre-treated P. australis at ISR of 1:1, 2:1 and 4:1 (Fig. 2a, Table 4). Moreover, the digestion of pre-treated P. australis at ISR 1:1 showed significantly higher cumulative biogas production than that produced at ISR 2:1, and those two had produced higher cumulative biogas than that produced at ISR 4:1. Similarly, it is appeared from Fig. 2 (c) that the digestion of untreated P. australis showed the highest cumulative biogas production at ISR of 1:4 and 1:2 followed by ISR of 1:1, while the lowest cumulative biogas production was observed at ISR of 2:1 and 4:1 (Table 4).
Cumulative biogas production plots of experimental (measured) and modified-Gompertz (predicted) data from pre-treated P. australis (a) and untreated P. australis (c) at different ISR, (
 ,
,
 ) 4:1, (
) 4:1, (
 ,
,
 ) 2:1, (
) 2:1, (
 ,
,
 ) 1:1, (
) 1:1, (
 ,
,
 ) 1:2, (
) 1:2, (
 ,
,
 ) 1:4, and (
) 1:4, and (
 ,
,
 ) inoculum control; and biogas production rate from pre-treated P. australis (b) and untreated P. australis (d) at different ISR, (
) inoculum control; and biogas production rate from pre-treated P. australis (b) and untreated P. australis (d) at different ISR, (
 ) 4:1, (
) 4:1, (
 ) 2:1, (
) 2:1, (
 ) 1:1, (
) 1:1, (
 ) 1:2, (
) 1:2, (
 ) 1:4, and (
) 1:4, and (
 ) inoculum control, over 32 days digestion period. Data represent mean ± standard deviation
) inoculum control, over 32 days digestion period. Data represent mean ± standard deviation
This increase in cumulative biogas production at ISR of 1:2 and 1:4 could be attributed to the rise in the substrate proportion, which contributed to providing more carbohydrates (such as cellulose and hemicellulose) that may be degraded by microbial activity into volatile fatty acids (VFAs), which in turn converted by methanogens into biogas. However, the VFAs production from digestion of pre-treated P. australis at ISR of 1:4 may exceed methanogens' consumption capability, which may result in a slight accumulation of VFAs and increased pH in the systems. These conditions may lead to a minor inhibition of microbial consortia and a slowdown in biogas production compared to other ISR, as shown in Fig. 2a [22, 37]. Consequently, the digestion of pre-treated P. australis using ISR of 1:2 is more suitable because it can enhance system stability and avoid the effects of acidification.
On the other hand, the low degradation of untreated P. australis due to the resistance of lignin to hydrolysis enzymes and limiting their accessibility to cellulose and hemicellulose could lead to producing a low amount of VFA, which corresponds to the consumption ability of methanogens bacteria [38]. Therefore, it can be observed from Fig. 2c that the digestion of untreated P. australis at ISR of 1:4 was stable and showed higher biogas production than other ISR.
Liew et al. [39] reported that the anaerobic digestion of four lignocellulosic substrates (corn stover, wheat straw, leaves, and yard waste) exhibited the highest methane production at ISR of 1:2 (81.2, 66.9, 55.4, and 40.8 mL/g VS, respectively) compared to ISR of 1:3, 1:4, and 1:5. Where the methane production decreased by 35–40% for corn stover and leaves when the ISR changed from 1:2 to 1:4, while decreased by 10–20% for wheat straw and yard waste. Similarly, Xu et al. [40] found that the anaerobic digestion of corn stover at ISR 1:2 produced higher methane production (238.5 mL/g VS) than that at ISR 1:4 and ISR 1:6 (199.6 and 120.0 mL/g VS respectively). Raposo et al. [27] found that the percentages of methane in the biogas produced from the digestion of fodder corn were increased from 54 to 59% with a reduction of ISRs from 3:1 to 1:1.
As shown in Fig. 2a, the predicted cumulative biogas plots of the modified Gompertz model are compatible with the experimental plots. The maximum cumulative biogas output of the model was obtained from pre-treated P. australis at ISR of 1:2 and 1:4 (78.95 and 76.03 mL/g VS added, respectively), while it was lesser at ISR of 1:1, 2:1 and 4:1 (53.70, 37.93, and 27.99 mL/g VS added, respectively). Similarly, the predicted cumulative biogas data from untreated P. australis were higher at ISR of 1:4 and 1:2 (28.53 and 24.79 mL/g VS added, respectively) than at ISR of 1:1, 2:1, and 4:1 (19.94, 14.36, and 13.70 mL/g VS added, respectively), as shown in Fig. 2c.
Figure 2b shows that the biogas production rate from the digestion of pre-treated P. australis at ISR 4:1, 2:1, 1:1, and 1:2 was high at the beginning of digestion, but it declined significantly after around 15 days. During this period, the digestion of pre-treated P. australis at ISR 1:2 showed the highest biogas production rate. Besides, the biogas production rate from ISR 1:4 was lower than other ISR at the initial stage of digestion, but it increased to become the highest after 15 days. This may indicate that a slight inhibition occurred at the initial stage of digestion, possibly due to the accumulation of VFAs produced, which may cause an increase in the acidity in the systems. Thus, using an ISR of 1:2 can be beneficial at a large-scale compared ISR of 1:4 because it helps to digest more materials in less time (around 15th days) and thus obtain larger quantities of biogas.
Similarly, the biogas production rate from the digestion of untreated P. australis was higher at ISR 1:2 and 1:4 than that from the other ISR during the digestion period (Fig. 2d). Therefore, the use of ISR 1:2 may consider the best option to enhance biogas production from the digestion of pre-treated P. australis and using ISR 1:2 or 1:4 to promote biogas production from the digestion of untreated P. australis.
Optimal Concentration of NaOH for Pre-treatment of P. australis to Enhance Biogas Production
As shown in Fig. 3a, the cumulative biogas production from pre-treated P. australis at various NaOH concentrations (0.5, 1, 2, and 4%) was significantly higher (p < 0.05) than that produced from untreated P. australis (Table 5). This may be due to the low digestibility of untreated P. australis because of the lignin recalcitrance to hydrolysis enzymes, which impeded them from reach to cellulose fibre. This may cause a decrease in the amount of VFAs produced from the degradation of untreated P. australis substrate, thus reducing biogas production. Besides that, it is observed that the digestion of pre-treated P. australis with 1% NaOH concentration showed significantly higher cumulative biogas production than pre-treated P. australis with 0.5% NaOH concentration. As well as the cumulative biogas production from the digestion of pre-treated P. australis with 2% and 4% NaOH concentration was significantly higher (p < 0.05) than that produced from pre-treated P. australis with 0.5% and 1% NaOH concentration. However, no significant difference was observed between the cumulative biogas produced from pre-treated P. australis with 2% and 4% NaOH (Table 5).
Cumulative biogas production plots of experimental (measured) and modified-Gompertz (predicted) data (a) from inoculum control (
 ,
,
 ), pre-treated P. australis with different NaOH concentrations (0.5% (
), pre-treated P. australis with different NaOH concentrations (0.5% (
 ,
,
 ), 1% (
), 1% (
 ,
,
 ), 2% (
), 2% (
 ,
,
 ), and 4% (
), and 4% (
 ,
,
 )) and untreated P. australis (
)) and untreated P. australis (
 ,
,
 ); and biogas production rate (b) from inoculum control (
); and biogas production rate (b) from inoculum control (
 ), pre-treated P. australis with different NaOH concentrations (0.5% (
), pre-treated P. australis with different NaOH concentrations (0.5% (
 ), 1% (
), 1% (
 ), 2% (
), 2% (
 ), and 4% (
), and 4% (
 )) and untreated P. australis (
)) and untreated P. australis (
 ), over 24 days digestion period. Data represent mean ± standard deviation
), over 24 days digestion period. Data represent mean ± standard deviation
This increase in biogas production as the NaOH concentration used for pre-treatment of P. australis increased (from 0.5 to 4%) can be attributed to the increased lignin removal from P. australis substrate, which enhances the bioaccessibility to cellulose component [7, 41], thus increase the biodegradation performance of the substrate and promote biogas production [42].
The study conducted by Zhu et al. [43] showed that the use of 5% NaOH for pre-treatment of corn stover at ambient temperature (20 ± 0.5 °C) for 24 h presented higher biogas production (372.4 mL/g VS) compared to that produced from the digestion of corn stover that pre-treated with 1%, 2.5%, and 7.5% NaOH concentrations (266.8 and 275.9 mL/g VS, respectively). Xue et al. [44] used NaOH at concentrations of 0%, 1%, 3%, 5%, and 8% in the pre-treatment of Miscanthus reed. They found that 8% NaOH was the best concentration for pre-treatment among the other NaOH concentrations, which increased the biogas production by 56.92%. The 1% NaOH concentration showed little effect on methane production, while methane production increased with the increasing NaOH concentration to achieve the highest level at 8% NaOH concentration (135.51 mL/g VS). Antonopoulou et al. [41] found that the increased NaOH concentration for pre-treatment of grass lawn waste (2, 10 and 20 g NaOH/100 g TS) led to improved substrate digestibility and increased methane production (389.0 ± 7.0, 397.7 ± 12.2 and 414.8 ± 26.5 mL CH4/g VS, respectively).
Figure 3a shows that the estimated cumulative biogas output of the modified Gompertz model is consistent with the experimental results. The highest cumulative biogas output of the model was obtained from P. australis pre-treated with 2% and 4% NaOH (69.28 and 75.49 mL/g VS added, respectively). In comparison, the lowest cumulative biogas output of the model was achieved from P. australis pre-treated with 1% and 0.5% NaOH and untreated P. australis (56.22, 44.07, and 17.62 mL/g VS added, respectively).
As shown in Fig. 3b, the digestion of the pre-treated P. australis with 0.5, 1 and 4% NaOH concentration exhibited the maximum biogas production rate on the first day of digestion (6.16, 6.81, and 7.13 mL/g VS added/day), while the digestion of the pre-treated P. australis with 2% NaOH began to produce 7.13 mL/g VS added/day on first day and rose to reach a maximum rate of 7.78 mL/g VS added/day on day three. However, the biogas production rate from the digestion of the pre-treated P. australis with 2 and 4% NaOH concentration remained for the first nine days in the range of 3.24–7.78 and 3.56–7.13 mL/g VS added/day, respectively, while the range was 2.27–6.16 and 2.59–6.81 mL/g VS added/day from the digestion of the pre-treated P. australis with 2 and 4% NaOH concentration, respectively. This may indicate more hydrolysis performance for P. australis substrate pre-treated with more NaOH concentration owing to enhancing lignin removal. Consequently, the digestion of P. australis pre-treated with 2% and 4% NaOH concentration can provide more biogas than that from P. australis pre-treated with 0.5% or 1% NaOH concentration within the same digestion period. Therefore, using 2% or 4% NaOH concentration is considered more effective for the pre-treatment of P australis substrate. However, since there was no significant difference between cumulative biogas produced from the digestion of pre-treated P. australis with 2% NaOH and 4% NaOH, and to increase the economic feasibly of pre-treatment, a concentration of 2% NaOH will be adopted in our future study for pre-treatment of P. australis biomass.
Optimal Incubation Time (12, 24, 48, 72, 96, 120 h) for Treatment of P. australis with 2% NaOH Concentration
The cumulative biogas produced from P. australis pre-treated with 2% NaOH at different incubation times (12, 24, 48, 72, 96, and 120 h) were relatively convergent during most of the digestion period (Fig. 4a). At the end of digestion period, no significant differences were observed in the cumulative biogas produced from the digestion of P. australis pre-treated with 2% NaOH at incubation times of 72, 96 and 120 h (Table 6). However, the cumulative biogas produced from the digestion of P. australis pre-treated at these three incubation times was significantly higher than that produced from P. australis pre-treated with 2% NaOH at incubation times of 12, 24, and 48 h (Table 6). This could have happened because the prolonged incubation time of pre-treatment resulted in higher lignin solubilization and removal, thus increasing cellulose availability for hydrolytic enzymes, which ultimately leads to increased biogas production [43, 45].
Cumulative biogas production plots of experimental (measured) and modified-Gompertz (predicted) data (a) from inoculum control (
 ,
,
 ) and P. australis pre-treated at different incubation times (12 h (
) and P. australis pre-treated at different incubation times (12 h (
 ,
,
 ), 24 h (
), 24 h (
 ,
,
 ), 48 h (
), 48 h (
 ,
,
 ), 72 h (
), 72 h (
 ,
,
 ), 96 h (
), 96 h (
 ,
,
 ), and 120 h (
), and 120 h (
 ,
,
 )), and biogas production rate (b) from inoculum control (
)), and biogas production rate (b) from inoculum control (
 ) and P. australis pre-treated at different incubation times, 12 h (
) and P. australis pre-treated at different incubation times, 12 h (
 ), 24 h (
), 24 h (
 ), 48 h (
), 48 h (
 ), 72 h (
), 72 h (
 ), 96 h (
), 96 h (
 ) and 120 h (
) and 120 h (
 ), over 25 days digestion period. Data points represent mean ± standard deviation
), over 25 days digestion period. Data points represent mean ± standard deviation
Similar results were found in other studies; for example, Zheng et al. [46] reported that biogas production increased by 72.9% when the corn stover was pre-treated with 2% NaOH for 72 h at ambient temperature (20 °C). Ewunie et al. [44] reported a maximum methane increment of 40.23% (353.90 mL/ g VS) was achieved from Jatropha press cake substrate that was pre-treated using 7.32% NaOH at 35.86 °C for 54.05 h. Chandra et al. [47] found that pre-treatment of wheat straw with 4% NaOH at 37 °C for 96 h achieved an 87.5% increase in biogas production compared to untreated wheat straw.
Figure 4a shows the output of the modified Gompertz model. The predicted model data fits well with the actual experimental results. The model data showed higher cumulative biogas output from P. australis pre-treated with 2% NaOH at incubation times of 72, 96 and 120 h (70.71, 69.47, and 69.61 mL/g VS added, respectively) than that pre-treated with 2% NaOH at incubation times of 12, 24, and 48 h (63.17, 65.17, and 67.04 mL/g VS added, respectively).
Similarly, it is observed that the biogas production rate from the digestion of P. australis pre-treated at incubation times of 12, 24, 48, 72, 96 and 120 h was relatively similar in the initial period of digestion (Fig. 4b). However, the digestion of P. australis pre-treated for 72, 96 and 120 h showed the highest biogas production rate until the end of the digestion period. Hence, using long incubation time for pre-treatment of P. australis substrate can be adopted to promote biogas production.
Conclusion
The use of small particle size (< 1 mm) helps increase homogeneity and interaction with microorganisms within the reactors. Since the highest biogas production was achieved from P. australis substrate that digested at ISR 1:2 and 1:4, pre-treated with 2% and 4% NaOH concentration, and pre-treated for the duration of 72, 96 and 120 h, with no significant differences in biogas production were detected. Thus, the digestion of pre-treated P. australis substrate with 2% NaOH for 72 h at an ISR of 1:2 could be practical and more feasible for applying in continuous anaerobic digesters at the pilot or full scale, because it reduces pre-treatment requirements and helps pre-treatment larger quantities of P. australis in a shorter period, in addition to doubling the amount of biogas that can be produced since the amount of substrate at ISR 1:2 is half that at ISR 1:4. In general, establishing these optimal parameters could provide guidance and support for future work to develop process configurations in continuous AD systems.
Change history
26 November 2023
The original version of this paper was updated to present the colored symbols in the figure captions.
References
Baute K (2015) Tall grass biomass for biogas: investigating the use of Phragmites australis (Cav.) Trin. ex. Steud.(Common Reed) as an energy feedstock in Ontario, Canada. Doctoral dissertation, University of Guelph. http://hdl.handle.net/10214/9114
Deublein D, Steinhauser A (2011) Biogas from waste and renewable resources: an introduction. John Wiley & Sons
Barbanti L, Di Girolamo G, Grigatti M et al (2014) Anaerobic digestion of annual and multi-annual biomass crops. Ind Crops Prod 56:137–144. https://doi.org/10.1016/J.INDCROP.2014.03.002
International Energy Agency (2020) Outlook for biogas and biomethane: prospects for organic growth. International Energy Agency, Paris
Massé D, Gilbert Y, Savoie P et al (2010) Methane yield from switchgrass harvested at different stages of development in Eastern Canada. Bioresour Technol 101:9536–9541. https://doi.org/10.1016/J.BIORTECH.2010.07.018
Parawira W, Murto M, Zvauya R, Mattiasson B (2004) Anaerobic batch digestion of solid potato waste alone and in combination with sugar beet leaves. Renew Energy 29:1811–1823. https://doi.org/10.1016/J.RENENE.2004.02.005
Olatunji KO, Ahmed NA, Ogunkunle O (2021) Optimization of biogas yield from lignocellulosic materials with different pretreatment methods: a review. Biotechnol Biofuels 14:1–34. https://doi.org/10.1186/S13068-021-02012-X
Hashemi B, Sarker S, Lamb JJ, Lien KM (2021) Yield improvements in anaerobic digestion of lignocellulosic feedstocks. J Clean Prod 288:125447. https://doi.org/10.1016/J.JCLEPRO.2020.125447
Allen E, Wall DM, Herrmann C, Murphy JD (2016) A detailed assessment of resource of biomethane from first, second and third generation substrates. Renew Energy 87:656–665. https://doi.org/10.1016/J.RENENE.2015.10.060
Song Y, Jain AK, Landuyt W et al (2015) Estimates of biomass yield for perennial bioenergy grasses in the USA. Bioenergy Res 8:688–715. https://doi.org/10.1007/S12155-014-9546-1
Allirand JM, Gosse G (1995) An above-ground biomass production model for a common reed (Phragmites communis Trin.) stand. Biomass Bioenergy 9:441–448. https://doi.org/10.1016/0961-9534(95)00042-9
Köbbing JF, Thevs N, Zerbe S (2013) The utilisation of reed (Phragmites australis): a review. Mires Peat 13:1–14
Demko J, Machava J, Saniga M (2017) Energy production analysis of Common Reed - Phragmites australis (Cav.) Trin. Folia Oecologica 44:107–113. https://doi.org/10.1515/FOECOL-2017-0013
Brix H (1989) Gas exchange through dead culms of reed, Phragmites australis (Cav.) Trin. ex Steudel. Aquat Bot 35:81–98. https://doi.org/10.1016/0304-3770(89)90069-7
Hendriks ATWM, Zeeman G (2009) Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour Technol 100:10–18. https://doi.org/10.1016/J.BIORTECH.2008.05.027
Kulichkova GI, Ivanova TS, Köttner M et al (2020) Plant feedstocks and their biogas production potentials. Open Agric J 14:219–234. https://doi.org/10.2174/1874331502014010219
Banu JR, Sugitha S, Kavitha S et al (2021) Lignocellulosic biomass pretreatment for enhanced bioenergy recovery: effect of lignocelluloses recalcitrance and enhancement strategies. Front Energy Res 9:646057. https://doi.org/10.3389/FENRG.2021.646057/BIBTEX
Barman DN, Haque MA, Kang TH et al (2013) Effect of mild alkali pretreatment on structural changes of reed (Phragmites communis Trinius) straw. Environ Technol 35:232–241. https://doi.org/10.1080/09593330.2013.824009
Neves L, Oliveira R, Alves MM (2004) Influence of inoculum activity on the bio-methanization of a kitchen waste under different waste/inoculum ratios. Process Biochem 39:2019–2024. https://doi.org/10.1016/J.PROCBIO.2003.10.002
Motte JC, Escudié R, Bernet N et al (2013) Dynamic effect of total solid content, low substrate/inoculum ratio and particle size on solid-state anaerobic digestion. Bioresour Technol 144:141–148. https://doi.org/10.1016/J.BIORTECH.2013.06.057
Kassongo J, Shahsavari E, Ball AS (2022) Substrate-to-inoculum ratio drives solid-state anaerobic digestion of unamended grape marc and cheese whey. Plos One 17:e0262940. https://doi.org/10.1371/JOURNAL.PONE.0262940
Makhura E, Muzenda E, Lekgoba T (2020) Effect of substrate to inoculum ratio on biogas yield. J Clean Energy Technol 8:16–19
Gandhi BP, Otite SV, Fofie EA et al (2022) Kinetic investigations into the effect of inoculum to substrate ratio on batch anaerobic digestion of simulated food waste. Renew Energy 195:311–321. https://doi.org/10.1016/J.RENENE.2022.05.134
Tran VG, Chu CY, Unpaprom Y et al (2021) Effects of substrate concentration and hydraulic retention time on hydrogen production from common reed by enriched mixed culture in continuous anaerobic bioreactor. Int J Hydrogen Energy 46:14036–14044. https://doi.org/10.1016/J.IJHYDENE.2020.07.092
Dubrovskis V, Kazulis V (2012) Biogas production potential from reeds. European Association for the Development of Renewable Energies, Environment and Power Quality (EA4EPQ) International Conference on Renewable Energies and Power Quality (ICREPQ’12) Santiago de Compostela (Spain), 28th to 30th March. https://doi.org/10.24084/repqj10.515
Kafle GK, Bhattarai S, Kim SH, Chen L (2014) Effect of feed to microbe ratios on anaerobic digestion of Chinese cabbage waste under mesophilic and thermophilic conditions: Biogas potential and kinetic study. J Environ Manage 133:293–301. https://doi.org/10.1016/J.JENVMAN.2013.12.006
Raposo F, Banks CJ, Siegert I et al (2006) Influence of inoculum to substrate ratio on the biochemical methane potential of maize in batch tests. Process Biochem 41:1444–1450. https://doi.org/10.1016/J.PROCBIO.2006.01.012
Xiao X, Zhang R, He Y et al (2013) Influence of particle size and alkaline pretreatment on the anaerobic digestion of corn stover. BioResources 8:5850–5860
Liu G, Zhang R, El-Mashad HM, Dong R (2009) Effect of feed to inoculum ratios on biogas yields of food and green wastes. Bioresour Technol 100:5103–5108. https://doi.org/10.1016/J.BIORTECH.2009.03.081
APHA (2005) Standard methods for the examination of water and wastewater. American Public Health Association, Washington, DC, USA, p 21
Ben Khedher N, Lattieff FA, Mahdi JM et al (2022) Modeling of biogas production and biodegradability of date palm fruit wastes with different moisture contents. J Clean Prod 375:134103. https://doi.org/10.1016/J.JCLEPRO.2022.134103
Kratky L, Jirout T (2011) Biomass size reduction machines for enhancing biogas production. Chem Eng Technol 34:391–399. https://doi.org/10.1002/CEAT.201000357
Dai X, Hua Y, Dai L, Cai C (2019) Particle size reduction of rice straw enhances methane production under anaerobic digestion. Bioresour Technol 293:122043. https://doi.org/10.1016/J.BIORTECH.2019.122043
Sharma SK, Mishra IM, Sharma MP, Saini JS (1988) Effect of particle size on biogas generation from biomass residues. Biomass 17:251–263. https://doi.org/10.1016/0144-4565(88)90107-2
Mshandete A, Björnsson L, Kivaisi AK et al (2006) Effect of particle size on biogas yield from sisal fibre waste. Renew Energy 31:2385–2392. https://doi.org/10.1016/J.RENENE.2005.10.015
Zborowska M, Waliszewska H, Waliszewska B et al (2021) Conversion of carbohydrates in lignocellulosic biomass after chemical pretreatment. Energies 15:254. https://doi.org/10.3390/EN15010254
Xu F, Li Y (2012) Solid-state co-digestion of expired dog food and corn stover for methane production. Bioresour Technol 118:219–226. https://doi.org/10.1016/J.BIORTECH.2012.04.102
Ma X, Jiang T, Chang J et al (2019) Effect of substrate to inoculum ratio on biogas production and microbial community during hemi-solid-state batch anaerobic co-digestion of rape straw and dairy manure. Appl Biochem Biotechnol 189:884–902. https://doi.org/10.1007/S12010-019-03035-9/FIGURES/5
Liew LN, Shi J, Li Y (2012) Methane production from solid-state anaerobic digestion of lignocellulosic biomass. Biomass Bioenergy 46:125–132. https://doi.org/10.1016/J.BIOMBIOE.2012.09.014
Xu F, Shi J, Lv W et al (2013) Comparison of different liquid anaerobic digestion effluents as inocula and nitrogen sources for solid-state batch anaerobic digestion of corn stover. Waste Manag 33:26–32. https://doi.org/10.1016/J.WASMAN.2012.08.006
He Y, Pang Y, Liu Y et al (2008) Physicochemical characterization of rice straw pretreated with sodium hydroxide in the solid state for enhancing biogas production. Energy Fuels 22:2775–2781. https://doi.org/10.1021/EF8000967/ASSET/IMAGES/LARGE/EF-2008-000967_0012.JPEG
Antonopoulou G, Vayenas D, Lyberatos G (2020) Biogas production from physicochemically pretreated grass lawn waste: comparison of different process schemes. Molecules 25:296. https://doi.org/10.3390/MOLECULES25020296
Zhu J, Wan C, Li Y (2010) Enhanced solid-state anaerobic digestion of corn stover by alkaline pretreatment. Bioresour Technol 101:7523–7528. https://doi.org/10.1016/J.BIORTECH.2010.04.060
Xue Y, Li Q, Gu Y et al (2020) Improving biodegradability and biogas production of miscanthus using a combination of hydrothermal and alkaline pretreatment. Ind Crops Prod 144:111985. https://doi.org/10.1016/J.INDCROP.2019.111985
Ewunie GA, Morken J, Yigezu ZD (2021) Alkaline and co-digestion pretreatments: process optimization for enhancing the methane yield of Jatropha press cake. Biomass Convers Biorefin 11:971–988. https://doi.org/10.1007/S13399-020-00732-Y/TABLES/9
Zheng M, Li X, Li L et al (2009) Enhancing anaerobic biogasification of corn stover through wet state NaOH pretreatment. Bioresour Technol 100:5140–5145. https://doi.org/10.1016/J.BIORTECH.2009.05.045
Chandra R, Takeuchi H, Hasegawa T, Kumar R (2012) Improving biodegradability and biogas production of wheat straw substrates using sodium hydroxide and hydrothermal pretreatments. Energy 43:273–282. https://doi.org/10.1016/J.ENERGY.2012.04.029
Acknowledgements
The authors thank Mr. Christopher James Parry, the Director of Cockerham Green Energy, in the UK, for his invaluable support and assistance to obtain inoculum/digestates. We extend our thanks to Mr. Tim Gregson and Dr. Clare Benskin, from Lancaster University in the UK for providing technical support.
Funding
This work was sponsored and supported by the Iraqi Ministry of Higher Education and Scientific Research and Mosul University, Iraq.
Author information
Authors and Affiliations
Contributions
Ahmed R. Al-Iraqi: Conceptualization, Methodology, Investigation, physio-chemical analysis, Formal analysis, Visualization, Writing – original draft, Writing – review & editing. Kirk T. Semple: Conceptualization, Methodology, Investigation, Formal analysis, Validation, Visualization, Resources, Supervision, Writing – review & editing. Andrew M. Folkard: Conceptualization, Methodology, Resources, Visualization, Supervision, Writing – review & editing. Philip A. Barker: Conceptualization, Resources, Visualization, Supervision, Writing – review & editing. Bhushan P. Gandhi: Formal analysis, Writing – review & editing.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Al-Iraqi, A.R., Gandhi, B.P., Folkard, A.M. et al. Determine the Optimal Parameters for Biogas Production from Common Reed (Phragmites australis). Bioenerg. Res. 17, 1302–1314 (2024). https://doi.org/10.1007/s12155-023-10699-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-023-10699-z