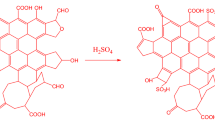
Abstract
The potential for electrochemical conversion of bio-oil was overlooked for a long period due to the poor electrical conductivity and unclear reaction mechanism. This study aimed to provide an insight into bio-oil of electrochemical conversion mechanism and product distribution. In this study, using walnut shell bio-oil with enhanced electrical conductivity by ammonium carbonate as raw material, electrolysis experiments were carried out using different electrolysis times by constant current method (20 mA) in H-type electrolytic cell at a stable temperature (0 °C). The results showed that phenols, aldehydes, and lignin were copolymerized; meanwhile, unsaturated fatty acids such as oleic acid underwent decarbonylation and decarboxylation. The hydrogen-releasing electrode reaction also occurred at the cathode during the conversion process. Besides, the properties of products were characterized by elemental analysis, GC-TCD/FID, GC/MS, 1H NMR, FTIR, and TG-FTIR. Solid products were of possibility for application in new materials owing to containing copolymers with lignin structure, and gas products could be utilized as gas fuels with the combustible gas (H2, CO, CH4, and olefins) content as 79.24%.

Graphical Abstract







Similar content being viewed by others
References
Wang C, Luo Z, Diao R, Zhu X (2019) Study on the effect of condensing temperature of walnut shells pyrolysis vapors on the composition and properties of bio-oil. Bioresour Technol 285:121370. https://doi.org/10.1016/j.biortech.2019.121370
Hayerly MR, Okoren KV, Brown RC (2016) Thermal stability of fractionated bio-oil from fast pyrolysis. Energy Fuel 30(11):9419–9426. https://doi.org/10.1021/acs.energyfuels.6b01606
Hu X, Gholizadeh M (2019) Biomass pyrolysis: a review of the process development and challenges from initial researches up to the commercialisation stage. J Energ Chem 39:109–143. https://doi.org/10.1016/j.jechem.2019.01.024
Saikia P, Gupta UN, Barman RS, Kataki R, Chutia RS, Baruah BP (2015) Production and characterization of bio-oil produced from Ipomoea carnea bio-weed. Bioenerg Res 8(3):1212–1223. https://doi.org/10.1007/s12155-014-9561-2
Kim JS (2015) Production, separation and applications of phenolic-rich bio-oil - a review. Bioresour Technol 178:90–98. https://doi.org/10.1016/j.biortech.2014.08.121
Yang W, Li X, Zhang D, Feng L (2017) Catalytic upgrading of bio-oil in hydrothermal liquefaction of algae major model components over liquid acids. Energy Convers Manag 154:336–343. https://doi.org/10.1016/j.enconman.2017.11.018
Agarwal A, Park S-J, Park J-H (2019) Upgrading of kraft lignin-derived bio-oil over hierarchical and nonhierarchical Ni and/or Zn/HZSM5 catalysts. Ind Eng Chem Res 58(51):22791–22803. https://doi.org/10.1021/acs.iecr.9b05348
Zhou M, Zeng Z, Zhu H, Xiao G, Xiao R (2014) Aqueous-phase catalytic hydrogenation of furfural to cyclopentanol over Cu-Mg-Al hydrotalcites derived catalysts: model reaction for upgrading of bio-oil. J Energ Chem 23(1):91–96. https://doi.org/10.1016/s2095-4956(14)60109-1
Xie H, Yu Q, Yao X, Duan W, Zuo Z, Qin Q (2015) Hydrogen production via steam reforming of bio-oil model compounds over supported nickel catalysts. J Energy Chem 24(3):299–308. https://doi.org/10.1016/s2095-4956(15)60315-1
Saber M, Nakhshiniev B, Yoshikawa K (2016) A review of production and upgrading of algal bio-oil. Renew Sust Energ Rev 58:918–930. https://doi.org/10.1016/j.rser.2015.12.342
Cheng S, Wei L, Julson J, Rabnawaz M (2017) Upgrading pyrolysis bio-oil through hydrodeoxygenation (HDO) using non-sulfided Fe-Co/SiO2 catalyst. Energy Convers Manag 150:331–342. https://doi.org/10.1016/j.enconman.2017.08.024
Pletcher D, Walsh FC (2012) Industrial electrochemistry. Springer Science & Business Media, Berlin
Newman J, Thomas-Alyea KE (2012) Electrochemical systems. John Wiley & Sons, Hoboken
Brett C (2004) Fundamentals of electrochemistry. In: Arnau Vives A (ed) Piezoelectric transducers and applications. Springer, Berlin Heidelberg, pp 185–194. https://doi.org/10.1007/978-3-662-05361-4_11
Bargon J, Mohmand S, Waltman RJ (1983) Electrochemical synthesis of electrically conducting polymers from aromatic-compounds. IBM J Res Dev 27(4):330–341. https://doi.org/10.1147/rd.274.0330
Frontana-Uribe BA, Little RD, Ibanez JG, Palma A, Vasquez-Medrano R (2010) Organic electrosynthesis: a promising green methodology in organic chemistry. Green Chem 12(12):2099–2119. https://doi.org/10.1039/c0gc00382d
Nikolaidis P, Poullikkas A (2017) A comparative overview of hydrogen production processes. Renew Sust Energ Rev 67:597–611. https://doi.org/10.1016/j.rser.2016.09.044
Chun YN, Song HW, Kim SC, Lim MS (2008) Hydrogen-rich gas production from biogas reforming using plasmatron. Energy Fuel 22(1):123–127. https://doi.org/10.1021/ef700302z
Fei H, Dong J, Arellano-Jimenez MJ, Ye G, Dong Kim N, Samuel EL, Peng Z, Zhu Z, Qin F, Bao J, Yacaman MJ, Ajayan PM, Chen D, Tour JM (2015) Atomic cobalt on nitrogen-doped graphene for hydrogen generation. Nat Commun 6:8668. https://doi.org/10.1038/ncomms9668
Nguyen T, Abdin Z, Holm T, Mérida W (2019) Grid-connected hydrogen production via large-scale water electrolysis. Energy Convers Manag 200:112108. https://doi.org/10.1016/j.enconman.2019.112108
Wang C, Ding H, Zhang Y, Zhu X (2020) Analysis of property variation and stability on the aging of bio-oil from fractional condensation. Renew Energy 148:720–728. https://doi.org/10.1016/j.renene.2019.10.159
Dhyani V, Bhaskar T (2018) A comprehensive review on the pyrolysis of lignocellulosic biomass. Renew Energy 129:695–716. https://doi.org/10.1016/j.renene.2017.04.035
Arnold S, Rodriguez-Uribe A, Misra M, Mohanty AK (2018) Slow pyrolysis of bio-oil and studies on chemical and physical properties of the resulting new bio-carbon. J Clean Prod 172:2748–2758. https://doi.org/10.1016/j.jclepro.2017.11.137
Marshall RJ, Walsh FC (1985) A review of some recent electrolytic cell designs. Surf Technol 24(1):45–77. https://doi.org/10.1016/0376-4583(85)90015-9
Zhu H, Wang L, Chen Y, Li G, Li H, Tang Y, Wan P (2014) Electrochemical depolymerization of lignin into renewable aromatic compounds in a non-diaphragm electrolytic cell. RSC Adv 4(56):29917–29924. https://doi.org/10.1039/c4ra03793f
Zhu WZ, Theliander H (2015) Precipitation of lignin from softwood black liquor: an investigation of the equilibrium and molecular properties of lignin. BioResources 10(1):1696–1714
Zhang L, Li S, Ding H, Zhu X (2019) Two-step pyrolysis of corncob for value-added chemicals and high-quality bio-oil: effects of alkali and alkaline earth metals. Waste Manag 87:709–718. https://doi.org/10.1016/j.wasman.2019.03.002
Vansoest PJ, Wine RH (1967) Use of detergents in analysis of fibrous feeds .4. determination of plant call-wall constituents. J Assoc Off Anal Chem 50(1):50-&
Abnisa F, Arami-Niya A, Daud WMAW, Sahu JN (2013) Characterization of bio-oil and bio-char from pyrolysis of palm oil wastes. Bioenerg Res 6(2):830–840. https://doi.org/10.1007/s12155-013-9313-8
Sluiter JB, Ruiz RO, Scarlata CJ, Sluiter AD, Templeton DW (2010) Compositional analysis of lignocellulosic feedstocks. 1. Review and description of methods. J Agric Food Chem 58(16):9043–9053. https://doi.org/10.1021/jf1008023
Zhu X, Luo Z, Diao R, Zhu X (2019) Combining torrefaction pretreatment and co-pyrolysis to upgrade biochar derived from bio-oil distillation residue and walnut shell. Energy Convers Manag 199:111970. https://doi.org/10.1016/j.enconman.2019.111970
Yaman E, Yargic AS, Ozbay N, Uzun BB, Kalogiannis KG, Stefanidis SD, Pachatouridou EP, Iliopoulou EF, Lappas AA (2018) Catalytic upgrading of pyrolysis vapours: effect of catalyst support and metal type on phenolic content of bio-oil. J Clean Prod 185:52–61. https://doi.org/10.1016/j.jclepro.2018.03.033
Long DH, Zhang J, Yang JH, Hu ZJ, Li TQ, Cheng G, Zhang R, Ling LC (2008) Preparation and microstructure control of carbon aerogels produced using m-cresol mediated sol-gel polymerization of phenol and furfural. New Carbon Mater 23(2):165–170. https://doi.org/10.1016/S1872-5805(08)60020-7
Wu D, Fu R (2006) Synthesis of organic and carbon aerogels from phenol–furfural by two-step polymerization. Microporous Mesoporous Mater 96(1–3):115–120. https://doi.org/10.1016/j.micromeso.2006.06.022
Zhang W, Ma Y, Wang C, Li S, Zhang M, Chu F (2013) Preparation and properties of lignin-phenol-formaldehyde resins based on different biorefinery residues of agricultural biomass. Ind Crop Prod 43:326–333. https://doi.org/10.1016/j.indcrop.2012.07.037
Blinkovsky AM, Dordick JS (1993) Peroxidase-catalyzed synthesis of lignin phenol copolymers. J Polym Sci A Polym Chem 31(7):1839–1846. https://doi.org/10.1002/pola.1993.080310722
Popp JL, Kirk TK, Dordick JS (1991) Incorporation of para-cresol into lignins via peroxidase-catalyzed copolymerization in nonaqueous media. Enzym Microb Technol 13(12):964–968. https://doi.org/10.1016/0141-0229(91)90118-t
Vazquez G, Gonzalez J, Freire S, Antorrena G (1997) Effect of chemical modification of lignin on the gluebond performance of lignin-phenolic resins. Bioresour Technol 60(3):191–198. https://doi.org/10.1016/s0960-8524(97)00030-8
Li FW, Ding SL, Li L, Gao C, Zhong Z, Wang SX, Li ZX (2016) Catalytic pyrolysis of model compounds and waste cooking oil for production of light olefins over La/ZSM-5 catalysts. 2016 International Conference on New Energy and Future Energy System (Nefes 2016) 40. Unsp 012072https://doi.org/10.1088/1755-1315/40/1/012072
Santillan-Jimenez E, Crocker M (2012) Catalytic deoxygenation of fatty acids and their derivatives to hydrocarbon fuels via decarboxylation/decarbonylation. J Chem Technol Biotechnol 87(8):1041–1050. https://doi.org/10.1002/jctb.3775
Thangalazhy-Gopakumar S, Adhikari S, Ravindran H, Gupta RB, Fasina O, Tu M, Fernando SD (2010) Physiochemical properties of bio-oil produced at various temperatures from pine wood using an auger reactor. Bioresour Technol 101(21):8389–8395. https://doi.org/10.1016/j.biortech.2010.05.040
Kundu A, Sahu JN, Redzwan G, Hashim MA (2013) An overview of cathode material and catalysts suitable for generating hydrogen in microbial electrolysis cell. Int J Hydrog Energy 38(4):1745–1757. https://doi.org/10.1016/j.ijhydene.2012.11.031
Hussain J, Jonsson H, Skulason E (2016) Faraday efficiency and mechanism of electrochemical surface reactions: CO2 reduction and H2 formation on Pt(111). Faraday Discuss 195:619–636. https://doi.org/10.1039/c6fd00114a
Zeng K, Zhang D (2010) Recent progress in alkaline water electrolysis for hydrogen production and applications. Prog Energy Combust Sci 36(3):307–326. https://doi.org/10.1016/j.pecs.2009.11.002
Ahmadi M, Macias EE, Jasinski JB, Ratnasamy P, Carreon MA (2014) Decarboxylation and further transformation of oleic acid over bifunctional, Pt/SAPO-11 catalyst and Pt/chloride Al2O3 catalysts. J Mol Catal A: Chem 386:14–19. https://doi.org/10.1016/j.molcata.2014.02.004
Tahir M, Amin NS (2015) Indium-doped TiO2 nanoparticles for photocatalytic CO2 reduction with H2O vapors to CH4. Appl Catal B-Environ 162:98–109. https://doi.org/10.1016/j.apcatb.2014.06.037
Joffres B, Nguyen MT, Laurenti D, Lorentz C, Souchon V, Charon N, Daudin A, Qignard A, Geantet C (2016) Lignin hydroconversion on MoS2-based supported catalyst: comprehensive analysis of products and reaction scheme. Appl Catal B-Environ 184:153–162. https://doi.org/10.1016/j.apcatb.2015.11.005
Hsiue GH, Shiao SJ, Wei HF, Kuo WJ, Sha YA (2001) Novel phosphorus-containing dicyclopentadiene-modified phenolic resins for flame-retardancy applications. J Appl Polym Sci 79(2):342–349. https://doi.org/10.1002/1097-4628(20010110)79:2<342::Aid-app180>3.3.Co;2-#
Wiedemeier DB, Abiven S, Hockaday WC, Keiluweit M, Kleber M, Masiello CA, McBeath AV, Nico PS, Pyle LA, Schneider MPW, Smernik RJ, Wiesenberg GLB, Schmidt MWI (2015) Aromaticity and degree of aromatic condensation of char. Org Geochem 78:135–143. https://doi.org/10.1016/j.orggeochem.2014.10.002
Long T, Li M, Chen YX, Zhu XF (2014) Study on evaporation characteristics of bio-oil and its compound models. BioResources 9(3):4242–4252
Zhu XF, Zhang YM, Ding HZ, Huang LR, Zhu XF (2018) Comprehensive study on pyrolysis and co-pyrolysis of walnut shell and bio-oil distillation residue. Energy Convers Manag 168:178–187. https://doi.org/10.1016/j.enconman.2018.05.012
Yang HP, Yan R, Chen HP, Lee DH, Zheng CG (2007) Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 86(12–13):1781–1788. https://doi.org/10.1016/j.fuel.2006.12.013
Liu Q, Wang S, Zheng Y, Luo Z, Cen K (2008) Mechanism study of wood lignin pyrolysis by using TG-FTIR analysis. J Anal Appl Pyrolysis 82(1):170–177. https://doi.org/10.1016/j.jaap.2008.03.007
Zhao LC, Guo MH, Li XD, Huang YP, Wu SH, Sun JJ (2017) Molar range detection based on sideband differential absorption spectroscopy with a concentrated reference. Anal Chem 89(24):13429–13433. https://doi.org/10.1021/acs.analchem.7b03722
Funding
This work was funded by the National Natural Science Foundation of China (No. 51676179).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yuan, X., Zhu, X., Zhang, L. et al. Fundamental Insights into Walnut Shell Bio-Oil Electrochemical Conversion: Reaction Mechanism and Product Properties. Bioenerg. Res. 14, 322–332 (2021). https://doi.org/10.1007/s12155-020-10155-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-020-10155-2