Abstract
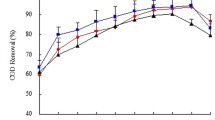
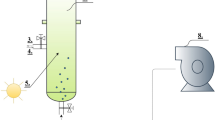
High-strength wastewaters after being digested for biogas production in anaerobic digesters still contain substantial nutrients and organics. The anaerobic digestates from four major industries in Thailand were tested with batch cultivation of Chlorella sp. for oil production potentials. Pig farm digestate was found most suitable as the growth medium generating 0.95 g/Lmedium (dry biomass), which was 1.16–3.06 times of other digestates tested. Considerable removals of nitrogen and phosphorus achieved were an added benefit to the goal of ultimate treatment of these wastewaters. Light intensity had strong influence on growth and heterotrophic metabolism up to 78 μmol/m2/s, while the dilution of digestate above 2.4× diminished growth potential and lipid production. A quadratic regression model was constructed to describe interaction of light intensity, dilution factor, and time of cultivation to lipid production with a satisfactory precision. Light intensity could influence fatty acid composition, although palmitic acid was found predominant at 47.1 %. The algae oil generated could potentially increase the total energy output from anaerobic digesters of a typical pig farm by 22 %.





Similar content being viewed by others
References
Thaibiogas. (2015) Thailand Energy Policy and Planning Office. 2015. http://www.thaibiogas.com/index2.php. Accessed 20 Mar 2015
Ahmad AL, Yasin NHM, Derek CJC, Lim JK (2011) Microalgae as a sustainable energy source for biodiesel production: a review. Renew Sust Energ Rev 15:584–593
Prajapati SK, Kaushik P, Malik A, Vijay VK (2013) Phycoremediation coupled production of algal biomass, harvesting and anaerobic digestion: possibilities and challenges. Bitechnol Adv 31:1408–1425
Mirsiaghi M, Reardon KF (2015) Conversion of lipid-extracted Nannochloropsis salina biomass into fermentable sugars. Algal Res 8:145–152
Vardon DR, Sharma BK, Blazina GV, Rajagopalan K, Strathmann TJ (2012) Thermochemical conversion of raw and defatted algal biomass via hydrothermal liquefaction and slow pyrolysis. Bioresour Technol 109:178–187
Abreu AP, Fernandes B, Vicente AA, Teixeira J, Dragone G (2012) Mixotrophic cultivation of Chlorella vulgaris using industrial dairy waste as organic carbon source. Bioresour Technol 118:61–66
Liang Y, Sarkany N, Cui Y (2009) Biomass and lipid productivities of Chlorella vulgaris under autotrophic, heterotrophic and mixotrophic growth conditions. Biotechnol Lett 31:1043–1049
Venkata MS, Rohit MV, Chiranjeevi P, Chandra R, Navaneeth B (2015) Heterotrophic microalgae cultivation to synergize biodiesel production with waste remediation: progress and perspectives. Bioresour Technol 184:169–178
Singh SP, Singh P (2015) Effect of temperature and light on the growth of algae species: a review. Renew Sust Energ Rev 50:431–444
George B, Pancha I, Desai C, Chokshi K, Paliwal C, Ghosh T, Mishra S (2014) Effects of different media composition, light intensity and photoperiod on morphology and physiology of freshwater microalgae Ankistrodesmus falcatus—a potential strain for bio-fuel production. Bioresour Technol 171:367–374
Passero M, Cragin B, Coats E, McDonald A, Feris K (2015) Dairy wastewaters for algae cultivation, polyhydroxyalkanoate reactor effluent versus anaerobic digester effluent. Bioenerg Res 8:1647–1660
Prajapati SK, Kumar P, Malik A, Vijay VK (2014) Bioconversion of algae to methane and subsequent utilization of digestate for algae cultivation: a closed loop bioenergy generation process. Bioresour Technol 158:174–180
Singh M, Reynolds DL, Das KC (2011) Microalgal system for treatment of effluent from poultry litter anaerobic digestion. Bioresour Technol 102:10841–10848
Xia A, Murphy JD (2016) Microalgal cultivation in treating liquid digestate from biogas systems. Trends Biotechnol 34:264–275
Dumrattana P, Tansakul P (2006) Cultivation of the hydrocarbon-rich alga, Botyococcus braunii in secondary treated effluent from a sea food processing plant. Songklanakarin J Sci Tech 28:99–105
APHA, AWWA, WPCF (2012) Standard methods for examination of water and wastewater. American Public Health Association, Washington, DC.
Gibson AM, Bratchell N, Roberts TA (1988) Predicting microbial growth: growth responses of salmonellae in a laboratory medium as affected by pH, sodium chloride and storage temperature. Int J Food Microbiol 6:155–178
McClure PJ, de W. Blackburn C, Cole MB, Curtis PS, Jones JE, Legan JD, Ogden ID, Peck MW, Roberts TA, Sutherland JP (1994) Modelling the growth, survival and death of microorganisms in foods: the UK food micromodel approach. Int J Food Microbiol 23:265–275
Folch J, Lees M, Stanley GHS (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 266:497–509
Collos Y, Berges JA (2004) Nitrogen metabolism in phytoplankton, Encyclopedia of Life Support Systems (EOLSS). EOLSS Publishers, Oxford
Hongyang S, Yalei Z, Chunmin W, Xuefei Z, Jinpeng L (2011) Cultivation of Chlorella pyrenoidosa in soybean processing wastewater. Bioresour Technol 102:9884–9890
Aslan S, Kapdan IK (2006) Batch kinetics of nitrogen and phosphorus removal from synthetic wastewater by algae. Ecol Eng 28:64–70
Khan M, Yoshida N (2008) Effect of L-glutamic acid on the growth and ammonium removal from ammonium solution and natural wastewater by Chlorella vulgaris NTM06. Bioresour Technol 99:572–582
Li Y, Han F, Xu H, Mu J, Chen D, Feng B, Zeng H (2014) Potential lipid accumulation and growth characteristic of the green alga Chlorella with combination cultivation mode of nitrogen (N) and phosphorus (P). Bioresour Technol 174:24–32
Lin L, Chan GYS, Jiang BL, Lan CY (2007) Use of ammoniacal nitrogen tolerant microalgae in landfill leachate treatment. Waste Manag 27:1376–1382
Jacob-Lopes E, Scoparo CHG, Lacerda LMCF, Franco TT (2009) Effect of light cycles (night/day) on CO2 fixation and biomass production by microalgae in photobioreactors. Chem Eng Process 48:306–310
Saifuddin N, Saltanat A, Refal H (2014) Enhancing the removal of phenolic compounds from palm oil mill effluent by enzymatic pre-treatment and microwave-assisted extraction. Chem Sci Trans 3:1083–1093
Li Y, Chen YF, Chen P, Min M, Zhou W, Martinez B, Zhu J, Ruan R (2011) Characterization of a microalga Chlorella sp. well adapted to highly concentrated municipal wastewater for nutrient removal and biodiesel production. Bioresour Technol 102:5138–5144
PCD (2015) Water Pollution Regulation, Pollution Control Department of Thailand. http://www.pcd.go.th/download/en_regulation.cfm?task=s3. Accessed 26 April 2015
Travieso L, Benítez F, Sánchez E, Borja R, Martín A, Colmenarejo MF (2006) Batch mixed culture of Chlorella vulgaris using settled and diluted piggery waste. Ecol Eng 28:158–165
Zhu L, Wang Z, Shu Q, Takala J, Hiltunen E, Feng P, Yuan Z (2013) Nutrient removal and biodiesel production by integration of freshwater algae cultivation with piggery wastewater treatment. Water Res 47:4294–4302
Somporn C, Kamtuo A, Theerakulpisut P, Siriamornpun S (2012) Effect of shading on yield, sugar content, phenolic acids and antioxidant property of coffee beans (Coffea Arabica L. cv. Catimor) harvested from north-eastern Thailand. J Sci Food Agric 92:1956–1963
Ye H, Jia S, Dai Y (2009) Growth characteristics of the cyanobacterium Nostoc flagelliforme in photoautotrophic, mixotrophic and heterotrophic cultivation. J Appl Phycol 21:127–133
Feng P, Deng Z, Fan L, Hu Z (2012) Lipid accumulation and growth characteristics of Chlorella zofingiensis under different nitrate and phosphate concentrations. J Biosci Bioeng 114:405–410
Chaiprapat S, Cheng JJ, Classen JJ, Liehr SK (2005) Role of internal nutrient storage in duckweed growth for swine wastewater treatment. T ASABE 48:2247–2258
Powell N, Shilton AN, Pratt S, Chisti Y (2008) Factors influencing luxury uptake of phosphorus by microalgae in waste stabilization ponds. Environ Sci Technol 42:5958–5962
Droop MR (1973) Some thoughts on nutrient limitation in algae 1. J Phycol 9:264–272
Xu H, Miao X, Wu Q (2006) High quality biodiesel production from a microalga Chlorella protothecoides by heterotrophic growth in fermenters. J Biotechnol 126:499–507
DEDE (2006) Technology for biogas production and utilization. Department of alternative Energy Development and Efficiency, Bangkok, Thailand
Chojnacka K, Marquez-Rocha FJ (2004) Kinetic and stoichiometric relationships of the energy and carbon metabolism in the culture of microalgae. Biotechnology 3:21–23
Farooq W, Lee YC, Ryu BG, Kim BH, Kim HS, Choi YE, Yang JW (2013) Two-stage cultivation of two Chlorella sp. strains by simultaneous treatment of brewery wastewater and maximizing lipid productivity. Bioresour Technol 132:230–238
Yang L, Tan X, Li D, Chu H, Zhou X, Zhang Y, Yu H (2015) Nutrients removal and lipids production by Chlorella pyrenoidosa cultivation using anaerobic digested starch wastewater and alcohol wastewater. Bioresour Technol 181:54–61
Ramos MJ, Fernández CM, Casas A, Rodríguez L, Pérez Á (2009) Influence of fatty acid composition of raw materials on biodiesel properties. Bioresour Technol 100:261–268
Acknowledgments
This research was financially supported by the Annual Research Budget of the Prince of Songkla University (PSU) contract no. ENG570183S and Graduate School of PSU, Thailand. The authors would like to recognize the full support for research facility of the Biogas and Biorefinery Research Laboratory, Faculty of Engineering, Prince of Songkla University, Thailand.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chaiprapat, S., Sasibunyarat, T., Charnnok, B. et al. Intensifying Clean Energy Production Through Cultivating Mixotrophic Microalgae from Digestates of Biogas Systems: Effects of Light Intensity, Medium Dilution, and Cultivating Time. Bioenerg. Res. 10, 103–114 (2017). https://doi.org/10.1007/s12155-016-9780-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-016-9780-9