Abstract
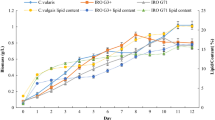
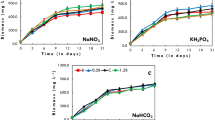
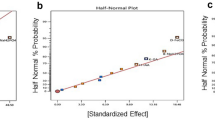
In the present study, process engineering strategy was applied to achieve lipid-rich biomass with high density of Chlorella sp. FC2 IITG under photoautotrophic condition. The strategy involved medium optimization, intermittent feeding of limiting nutrients, dynamic change in light intensity, and decoupling growth and lipid induction phases. Medium optimization was performed using combinations of artificial neural network or response surface methodology with genetic algorithm (ANN-GA and RSM-GA). Further, a fed-batch operation was employed to achieve high cell density with intermittent feeding of nitrate and phosphate along with stepwise increase in light intensity. Finally, mutually exclusive biomass and lipid production phases were decoupled into two-stage cultivation process: biomass generation in first stage under nutrient sufficient condition followed by lipid enrichment through nitrogen starvation. The key findings were as follows: (i) ANN-GA resulted in an increase in biomass titer of 157 % (0.95 g L−1) in shake flask and 42.8 % (1.0 g L−1) in bioreactor against unoptimized medium at light intensity of 20 μE m−2 s−1; (ii) further optimization of light intensity in bioreactor gave significantly improved biomass titer of 5.6 g L−1 at light intensity of 250 μE m−2 s−1; (iii) high cell density of 13.5 g L−1 with biomass productivity of 675 mg L−1 day−1 was achieved with dynamic increase in light intensity and intermittent feeding of limiting nutrients; (iv) finally, two-phase cultivation resulted in biomass titer of 17.7 g L−1 and total lipid productivity of 313 mg L−1 day−1 which was highest among Chlorella sp. under photoautotrophic condition.







Similar content being viewed by others
References
Raja R, Hemaiswarya S, Kumar NA, Sridhar S, Rengasamy R (2008) A perspective on the biotechnological potential of microalgae. Crit Rev Microbiol 34(2):77–88
Hu Q, Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M, Seibert M, Darzins A (2008) Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J 54:621–639
Rodolfi L, Zittelli GC, Bassi N, Padovani G, Biondi N, Bonini G, Tredici MR (2009) Microalgae for oil: strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol Bioeng 102(1):100–112
Lim DKY, Garg S, Timmins M, Zhang ESB, Thomas-Hall SR, Schuhmann H, Li Y, Schenk PM (2012) Isolation and evaluation of oil-producing microalgae from subtropical coastal and brackish waters. PLoS One 7(7):1–13
Muthuraj M, Kumar V, Palabhanvi B, Das D (2014) Evaluation of indigenous microalgal isolate Chlorella sp. FC2 IITG as a cell factory for biodiesel production and scale up in outdoor conditions. J Ind microb Biotechnol 41:499–511
Muthuraj M, Palabhanvi B, Misra S, Kumar V, Sivalingavasu K, Das D (2013) Flux balance analysis of Chlorella sp. FC2 IITG under photoautotrophic and heterotrophic growth conditions. Photosynth Res 118:167–179
De la Hoz SH, McCaffrey WC, Burrell RE, Ben-Zvi A (2012) Optimization of microalgal productivity using an adaptive, non-linear model based strategy. Bioresour Technol 104:537–546
Palabhanvi B, Belur PD (2013) Enhancing gallic acid content in green tea extract by using novel cell associated tannase of Bacillus massiliensis. J Food Biochem 37:528–535
Sathish T, Prakasham RS (2010) Enrichment of glutaminase production by Bacillus subtilis RSP-Glu in submerged cultivation based on neural network-genetic algorithm approach. J Chem Technol Biotechnol 85:50–58
Zafar M, Kumar S, Kumar S, Dhiman AK (2012) Artificial intelligence based modeling and optimization of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) production process by using Azohydromonas lata MTCC 2311 from cane molasses supplemented with volatile fatty acids: a genetic algorithm paradigm. Bioresour Technol 104:631–641
Whiteman JK, Kana EBG (2014) Comparative assessment of the artificial neural network and response surface modelling efficiencies for biohydrogen production on sugar cane molasses. Bioenerg Res 7:295–305
Wahidin S, Idris A, Shaleh SRM (2013) The influence of light intensity and photoperiod on the growth and lipid content of microalgae Nannochloropsis sp. Bioresour Technol 129:7–11
Wu YH, Yu Y, Hu HY (2014) Effects of initial phosphorous concentration and light intensity on biomass yield per phosphorous and lipid accumulation of Scenedesmus sp. LX1. Bioenerg Res. doi:10.1007/s12155-014-9411-2
Yeh K, Chang J (2011) Nitrogen starvation strategies and photobioreactor design for enhancing lipid production of a newly isolated microalga Chlorella vulgaris ESP-31: implications for biofuels. Biotechnol J 6:1358–1366
Bumbak F, Cook S, Zachleder V, Hauser S, Kovar K (2011) Best practices in heterotrophic high-cell-density microalgal processes: achievements, potential and possible limitations. Appl Microbiol Biotechnol 91:31–46
Kumar V, Muthuraj M, Palabhanvi B, Ghoshal AK, Das D (2014) Evaluation and optimization of two stage sequential in situ transesterification process for fatty acid methyl ester quantification from microalgae. Renew Energy 68:560–569
Mohamed MS, Tan JS, Mohamad R, Mokhtar MN, Ariff AB (2013) Comparative analyses of response surface methodology and artificial neural network on medium optimization for Tetraselmis sp. FTC209 grown under mixotrophic condition. Sci World J. doi:10.1155/2013/948940
Haider MA, Pakshirajan K, Singh A, Chaudhry S (2008) Artificial neural network-genetic algorithm approach to optimize media constituents for enhancing lipase production by a soil microorganism. Appl Biochem Biotechnol 144:225–235
Franco-Lara E, Link H, Weuster-Botz D (2006) Evaluation of artificial neural networks for modelling and optimization of medium composition with a genetic algorithm. Process Biochem 41:2200–2206
Thimijan RW, Heins RD (1982) Photometric, radiometric, and quantum light units of measure: a review of procedures for interconversion. Hortic Sci 18:818–822
Wybenga DR, Giorgio JD, Pileggi VJ (1971) Manual and automated methods for urea nitrogen measurement in whole serum. Clin Chem 17(9):891–895
Parsons TR, Maita Y, Lalli CM (1984) A manual of chemical and biological methods for seawater analysis. Pergamon Press Ltd, Great Britain
Zhang Y, Xu J, Yuan Z, Xu H, Yu Q (2010) Artificial neural network-genetic algorithm based optimization for the immobilization of cellulose on the smart polymer Eudragit L-100. Bioresour Technol 101:3153–3158
Zafar M, Kumar S, Kumar S (2010) Optimization of naphthalene biodegradation by a genetic algorithm based response surface methodology. Braz J Chem Eng 27:89–99
Sivapathasekaran C, Sen R (2013) Performance evaluation of an ANN-GA aided experimental modeling and optimization procedure for enhanced synthesis of marine biosurfactant in a stirred tank reactor. J Chem Technol Biotechnol 88:794–799
Wang J, Wan W (2009) Optimization of fermentative hydrogen production process using genetic algorithm based on neural network and response surface methodology. Int J Hydrogen Energy 34:255–261
Cheng K-C, Ren M, Ogden KL (2013) Statistical optimization of culture media for growth and lipid production of Chlorella protothecoides UTEX 250. Bioresour Technol 128:44–48
Bapat PM, Wangikar PP (2004) Optimization of rifamycin B fermentation in shake flask via a machine-learning based approach. Biotechnol Bioeng 86(2):201–208
Karemore A, Pal R, Sen R (2013) Strategic enhancement of algal biomass and lipid in Chlorococcum infusionumas bioenergy feedstock. Algal Res 2:113–121
Pruvost J, Van Vooren G, Le Gouic B, Couzinet-Mossion A, Legrand J (2011) Systematic investigation of biomass and lipid productivity by microalgae in photobioreactors for biodiesel application. Bioresour Technol 102:150–158
Barsanti L, Gualtieri P (2006) Algae: anatomy, biochemistry and biotechnology. CRC, Boca Raton
Sforza E, Simionato D, Giacometti GM, Bertucco A, Morosinotto T (2012) Adjusted light and dark cycles can optimize photosynthetic efficiency in algae growing in photobioreactors. Plos One 7(6):1–10, e38975
Li Y, Fei X, Deng X (2012) Novel molecular insights into nitrogen starvation-induced triacylglycerols accumulation revealed by differential gene expression analysis in green algae Micractinium pusillum. Biomass Bioenergy 42:199–211
Zhang D, Xue S, Sun Z, Liang K, Wang L, Zhang Q, Cong W (2014) Investigation of continuous-batch mode of two-stage culture of Nannochloropsis sp. for lipid production. Bioprocess Biosyst Eng. doi:10.1007/s00449-014-1185-6
Hseih C, Wu W (2009) Cultivation of microalgae for oil production with a cultivation strategy of urea limitation. Bioresour Technol 100:3921–3926
Han F, Huang J, Li Y, Wang W, Wan M, Shen G, Wang J (2013) Enhanced lipid productivity of Chlorella pyrenoidosa through the culture strategy of semi-continuous cultivation with nitrogen limitation and pH control by CO2. Bioresour Technol 136:418–424
Kumar V, Muthuraj M, Palabhanvi B, Ghoshal AK, Das D (2014) High cell density lipid rich cultivation of a novel microalgal isolate Chlorella sorokiniana FC6 IITG in a single-stage fed-batch mode under mixotrophic condition. Bioresour Technol 170:115–124
Acknowledgments
Department of Biotechnology, India, is gratefully acknowledged for the financial support provided (No. BT/PR484/PBD/26/259/2011).
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 37 kb)
Rights and permissions
About this article
Cite this article
Muthuraj, M., Chandra, N., Palabhanvi, B. et al. Process Engineering for High-Cell-Density Cultivation of Lipid Rich Microalgal Biomass of Chlorella sp. FC2 IITG. Bioenerg. Res. 8, 726–739 (2015). https://doi.org/10.1007/s12155-014-9552-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-014-9552-3