Abstract
Objective
This study aims to elucidate differences in preoperative cerebral glucose metabolism between patients who did and did not become seizure free after unilateral mesial temporal lobe epilepsy (mTLE) surgery. We hypothesized that regional glucose metabolism on preoperative fluorodeoxyglucose positron emission tomography (FDG-PET) in patients with seizure-free outcomes differed from that in patients who were not seizure free after appropriate epilepsy surgery for unilateral mTLE. In this study, we compared preoperative FDG-PET findings between these two patient groups by applying a statistical analysis approach, with a voxel-based Asymmetry index (AI), to improve sensitivity for the detection of hypometabolism.
Methods
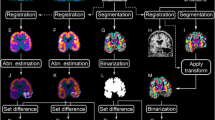
FDG-PET scans of 28 patients with medically refractory mTLE, of whom 17 achieved a seizure-free outcome (Engel class 1 a–b) during a postoperative follow-up period of at least 2 years, were analyzed retrospectively. Voxel values were adjusted by the AI method as well as the global normalization (GN) method. Two types of statistical analysis were performed. One was a voxel severity analysis with comparison of voxel values at the same coordinate, and the other was extent analysis with comparison of the number of significant voxels in the anatomical areas predefined with Talairach’s atlas.
Results
In the voxel severity analysis, significant hypometabolism restricted to the ipsilateral temporal tip and hippocampal area was detected in the postoperative seizure-free outcome group as compared to controls. No significant area was detected in the non-seizure-free group as compared to controls (family-wise error corrected, p < 0.05). With extent analysis using a low threshold, the extents of hypometabolism in the ipsilateral hippocampal, frontal and thalamic areas were larger in the seizure-free than in the non-seizure-free group (p = 0.01, 0.03 and 0.01, respectively.) On the other hand, in the contralateral frontal and thalamic areas, extents of hypometabolism were smaller in the seizure-free than in the non-seizure-free group (p = 0.01 and 0.01).
Conclusions
We found the preoperative distribution of hypometabolism to differ between the two patient groups. Severe hypometabolism restricted to the unilateral temporal lobe, with ipsilateral dominant hypometabolism including mild decreases, may support the existence of an epileptogenic focus in the unilateral temporal lobe and a favorable seizure outcome after mTLE surgery.



Similar content being viewed by others
References
Wiebe S, Blume WT, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345:311–8.
Salanova V, Markand O, Worth R. Longitudinal follow-up in 145 patients with medically refractory temporal lobe epilepsy treated surgically between 1984 and 1995. Epilepsia. 1999;40:1417–23.
Sokoloff L, Reivich M, Kennedy C, Des Rosiers MH, Patlak CS, Pettigrew KD, et al. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem. 1977;28:897–916.
Reivich M, Kuhl D, Wolf A, Greenberg J, Phelps M, Ido T, et al. The [18F]fluorodeoxyglucose method for the measurement of local cerebral glucose utilization in man. Circ Res. 1979;44:127–37.
Alavi A, Dann R, Chawluk J, Alavi J, Kushner M, Reivich M. Positron emission tomography imaging of regional cerebral glucose metabolism. Semin Nucl Med. 1986;16:2–34.
Henry TR, Babb TL, Engel J Jr, Mazziotta JC, Phelps ME, Crandall PH. Hippocampal neuronal loss and regional hypometabolism in temporal lobe epilepsy. Ann Neurol. 1994;36:925–7.
Vinton AB, Carne R, Hicks RJ, Desmond PM, Kilpatrick C, Kaye AH, et al. The extent of resection of FDG-PET hypometabolism relates to outcome of temporal lobectomy. Brain. 2007;130:548–60.
Matheja P, Kuwert T, Ludemann P, Weckesser M, Kellinghaus C, Schuierer G, et al. Temporal hypometabolism at the onset of cryptogenic temporal lobe epilepsy. Eur J Nucl Med. 2001;28:625–32.
Radtke RA, Hanson MW, Hoffman JM, Crain BJ, Walczak TS, Lewis DV, et al. Temporal lobe hypometabolism on PET: predictor of seizure control after temporal lobectomy. Neurology. 1993;43:1088–92.
Manno EM, Sperling MR, Ding X, Jaggi J, Alavi A, O’Connor MJ, et al. Predictors of outcome after anterior temporal lobectomy: positron emission tomography. Neurology. 1994;44:2331–6.
Theodore WH, Sato S, Kufta CV, Gaillard WD, Kelley K. FDG-positron emission tomography and invasive EEG: seizure focus detection and surgical outcome. Epilepsia. 1997;38:81–6.
Choi JY, Kim SJ, Hong SB, Seo DW, Hong SC, Kim BT, et al. Extratemporal hypometabolism on FDG PET in temporal lobe epilepsy as a predictor of seizure outcome after temporal lobectomy. Eur J Nucl Med Mol Imaging. 2003;30:581–7.
Henry TR, Mazziotta JC, Engel J Jr. Interictal metabolic anatomy of mesial temporal lobe epilepsy. Arch Neurol. 1993;50:582–9.
Hashiguchi K, Morioka T, Yoshida F, Kawamura T, Miyagi Y, Kuwabara Y, et al. Thalamic hypometabolism on 18FDG-positron emission tomography in medial temporal lobe epilepsy. Neurol Res. 2007;29:215–22.
Newberg AB, Alavi A, Berlin J, Mozley PD, O’Connor M, Sperling M. Ipsilateral and contralateral thalamic hypometabolism as a predictor of outcome after temporal lobectomy for seizures. J Nucl Med. 2000;41:1964–8.
Lee SK, Lee DS, Yeo JS, Lee JS, Kim YK, Jang MJ, et al. FDG-PET images quantified by probabilistic atlas of brain and surgical prognosis of temporal lobe epilepsy. Epilepsia. 2002;43:1032–8.
Soma T, Momose T, Takahashi M, Koyama K, Kawai K, Murase K, et al. Usefulness of extent analysis for statistical parametric mapping with asymmetry index using inter-ictal FGD-PET in mesial temporal lobe epilepsy. Ann Nucl Med. 2012;26:319–26.
Shimizu H, Kawai K, Sunaga S, Sugano H, Yamada T. Hippocampal transection for treatment of left temporal lobe epilepsy with preservation of verbal memory. J Clin Neurosci. 2006;13:322–8.
Engel J Jr, Van Ness PC, Rasmussen TB, Ojemann LM. Outcome with respect to epileptic seizures. In: Engel Jr J, editor. Surgical treatment of the epilepsies. New York: Raven Press; 1993. p. 609–21.
Fox PT, Mintun MA, Reiman EM, Raichle ME. Enhanced detection of focal brain responses using intersubject averaging and change-distribution analysis of subtracted PET images. J Cereb Blood Flow Metab. 1988;8:642–53.
Lancaster JL, Rainey LH, Summerlin JL, Freitas CS, Fox PT, Evans AC, et al. Automated labeling of the human brain: a preliminary report on the development and evaluation of a forward-transform method. Hum Brain Mapp. 1997;5:238–42.
Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, et al. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–31.
Rubin E, Dhawan V, Moeller JR, Takikawa S, Labar DR, Schaul N, et al. Cerebral metabolic topography in unilateral temporal lobe epilepsy. Neurology. 1995;45:2212–23.
Khan N, Leenders KL, Hajek M, Maguire P, Missimer J, Wieser HG. Thalamic glucose metabolism in temporal lobe epilepsy measured with 18F-FDG positron emission tomography (PET). Epilepsy Res. 1997;28:233–43.
Dupont P, Zaknun JJ, Maes A, Tepmongkol S, Vasquez S, Bal CS, et al. Dynamic perfusion patterns in temporal lobe epilepsy. Eur J Nucl Med Mol Imaging. 2009;36:823–30.
Benedek K, Juhasz C, Muzik O, Chugani DC, Chugani HT. Metabolic changes of subcortical structures in intractable focal epilepsy. Epilepsia. 2004;45:1100–5.
Guo Y, Gao F, Wang S, Ding Y, Zhang H, Wang J, et al. In vivo mapping of temporospatial changes in glucose utilization in rat brain during epileptogenesis: an 18F-fluorodeoxyglucose-small animal positron emission tomography study. Neuroscience. 2009;162:972–9.
Gale K. Subcortical structures and pathways involved in convulsive seizure generation. J Clin Neurophysiol. 1992;9:264–77.
Bouilleret V, Boyet S, Marescaux C, Nehlig A. Mapping of the progressive metabolic changes occurring during the development of hippocampal sclerosis in a model of mesial temporal lobe epilepsy. Brain Res. 2000;852:255–62.
Lambert MV, Robertson MM. Depression in epilepsy: etiology, phenomenology, and treatment. Epilepsia. 1999;40:S21–47.
Hosokawa T, Momose T, Kasai K. Brain glucose metabolism difference between bipolar and unipolar mood disorders in depressed and euthymic states. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:243–50.
Takaya S, Hanakawa T, Hashikawa K, Ikeda A, Sawamoto N, Nagamine T, et al. Prefrontal hypofunction in patients with intractable mesial temporal lobe epilepsy. Neurology. 2006;67:1674–6.
Van Bogaert P, Massager N, Tugendhaft P, Wikler D, Damhaut P, Levivier M, et al. Statistical parametric mapping of regional glucose metabolism in mesial temporal lobe epilepsy. Neuroimage. 2000;12:129–38.
Acknowledgments
We would like to thank the staff of our Nuclear Medicine Department and our secretaries, for their contributions to this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Takahashi, M., Soma, T., Kawai, K. et al. Voxel-based comparison of preoperative FDG-PET between mesial temporal lobe epilepsy patients with and without postoperative seizure-free outcomes. Ann Nucl Med 26, 698–706 (2012). https://doi.org/10.1007/s12149-012-0629-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-012-0629-9