Abstract
Objective
Positron emission tomography (PET) can be used to locate epileptic foci in patients with mesial temporal lobe epilepsy (MTLE) by measuring multiple parameters of the brain. We investigated a series of patients with MTLE using PET measurements of three parameters: the cerebral blood flow measured with [15O] H2O, the uptake of [18F] fluorodeoxyglucose (FDG), an index of the cerebral metabolism rate of glucose, and the distribution volume (DV) of [11C] flumazenil (FMZ), an index of the binding potential of central benzodiazepine receptor. We compared predictive values obtained from two methods: a voxel-based statistical analysis using statistical parametric mapping (SPM) and an asymmetry index obtained by placing regions of interest (ROIs) on PET images.
Methods
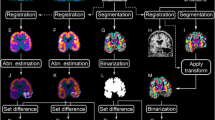
Preoperative PET data of 11 patients with surgically confirmed MTLE were retrospectively examined. In the voxel-based analysis, the PET data were analyzed using SPM99 by statistically comparing the voxel values of PET parameters between individual patients and the mean values of 12 normal volunteers. Voxels with values significantly lower than the normal control values were mapped on a standard brain atlas. In the ROI-based analysis, the asymmetry index was calculated to depict ROIs with abnormally decreased values when compared with the contralateral side.
Results
(1) Statistical parametric mapping and ROI analyses of the FDG uptake correctly determined epileptic temporal lobe in 73% and 82%, respectively. (2) The decreased DV of FMZ depicted by SPM revealed the mesial temporal pathology in 91%.
Conclusions
Positron emission tomography measurement of FDG uptake was most sensitive in detecting the side of the epileptic focus. On the other hand, SPM analysis of the DV of FMZ was the most sensitive method for delineating the actual epileptic focus.
Similar content being viewed by others
References
Henry TR, Chugani HT, Abou-Khalil BW, Theodore WH, Swartz BE. Positron emission tomography. In: Engel J Jr, editor. Surgical treatment of the epilepsies. 2nd ed. New York: Raven Press; 1993. p. 211–232.
Spencer SS, Bautista RED. Functional neuroimaging in localization of the ictal onset zone. In: Henry TR, Duncan JS, Berkovic SF, editors. Advances in neurology, Vol. 83. Functional imaging in the epilepsies. Philadelphia: Lipponcott Williams & Wilkins; 2000. p. 285–296.
Henry TR. PET: cerebral blood flow and glucose metabolism-presurgical localization. In: Henry TR, Duncan JS, Berkovic SF, editors. Advances in neurology, Vol. 83. Functional imaging in the epilepsies. Philadelphia: Lipponcott Williams & Wilkins; 2000. p. 105–120.
Engel J Jr, Henry TR, Risinger MW. The role of positron emission tomography in presurgical evaluation of temporal lobe epilepsy. In: Luders H, editor. Epilepsy surgery. New York: Raven Press; 1991. p. 231–241.
Duncan JS, Koepp MJ. PET: central benzodiazepine neuroreceptor mapping in localization-related epilepsies. In: Henry TR, Duncan JS, Berkovic SF, editors. Advances in neurology. Vol. 83. Functional imaging in the epilepsies. Philadelphia: Lipponcott Williams & Wilkins; 2000. p. 131–136.
Henry TR. Progress in epilepsy research: functional neuroimaging with positron emission tomography. Epilepsia 1996;37:1141–1154.
Lee DS, Lee JS, Kang KW, Jang MJ, Lee SK, Chung JK, et al. Disparity of perfusion and glucose metabolism of epileptogenic zones in temporal lobe epilepsy demonstrated by SPM/SPAM analysis on 15O water PET, [18F]FDG-PET, and [99mTc]-HMPAO SPECT. Epilepsia 2001;42:1515–1522.
Gaillard WD, Fazilat S, White S, Malow B, Sato S, Reeves P, et al. Interictal metabolism and blood flow are uncoupled in temporal lobe cortex of patients with complex partial epilepsy. Neurology 1995;45:1841–1847.
Leiderman DB, Balish M, Sato S, Kufta C, Reeves P, Gaillard WD, et al. Comparison of PET measurements of cerebral blood flow and glucose metabolism for the localization of human epileptic foci. Epilepsy Res 1992;13:153–157.
Ryvlin P, Bouvard S, Le Bars D, De Lamerie G, Gregoire MC, Kahane P, et al. Clinical utility of flumazenil-PET versus [18F]fluorodeoxyglucose-PET and MRI in refractory partial epilepsy: a prospective study in 100 patients. Brain 1998;121:2067–2081.
Savic I, Ingvar M, Stone-Elander S. Comparison of [11C]flumazenil and [18F]FDG as PET markers of epileptic foci. J Neurol Neurosurg Psychiatry 1993;56:615–621.
Engel J Jr, Van Ness PC, Rasmussen TB, Ojemann LM. Outcome with respect to epileptic seizure. In: Engel J Jr, editor. Surgical treatment of the epilepsies. 2nd ed. New York: Raven Press; 1993. p. 609–621.
Ardekani BA, Braun M, Hutton BF, Kanno I, Iida H. A fully automatic multimodality image registration algorithm. J Comput Assist Tomogr 1995;19:615–623.
Kuzniecky RI, Cascino GD, Palmini A, Jack CR Jr, Berkovic SF, Jackson GD, et al. Structural neuroimaging. In: Engel J Jr, editor. Surgical treatment of the epilepsies. 2nd ed. New York: Raven Press; 1993. p. 197–209.
Theodore WH. PET: cerebral blood flow and glucose metabolism-pathophysiology and drug effects. In: Henry TR, Duncan JS, Berkovic SF, editors. Advances in neurology, Vol. 83. Functional imaging in the epilepsies. Philadelphia: Lipponcott Williams & Wilkins; 2000. p. 121–130.
Takaya S, Hanakawa T, Hashikawa K, Ikeda A, Sawamoto N, Nagamine T, et al. Prefrontal hypofunction in patients with intractable mesial temporal lobe epilepsy. Neurology 2006;67:1674–1676.
Kim YK, Lee DS, Lee SK, Kim SK, Chung CK, Chang KH, et al. Differential features of metabolic abnormalities between medial and lateral temporal lobe epilepsy: quantitative analysis of 18F-FDG PET using SPM. J Nucl Med 2003;44:1006–1012.
Duarte PS, Zhuang H, Couturier O, Alavi A. Does semiquantitative analysis of FDG-PET have any additional value in the diagnosis of mesial temporal sclerosis? Arq Neuropsiquiatr 2001;59:871–874.
Newberg AB, Alavi A, Berlin J, Mozley PD, O’Connor M, Sperling M. Ipsilateral and contralateral thalamic hypometabolism as a predictor of outcome after temporal lobectomy for seizures. J Nucl Med 2000;41:1964–1968.
Hammers A, Koepp MJ, Labbe C, Brooks DJ, Thom M, Cunningham VJ, et al. Neocortical abnormalities of [11C]-flumazenil PET in mesial temporal lobe epilepsy. Neurology 2001;56:897–906.
Koepp MJ, Labbe C, Richardson MP, Brooks DJ, Van Paesschen W, Cunningham VJ, et al. Regional hippocampal [11C]flumazenil PET in temporal lobe epilepsy with unilateral and bilateral hippocampal sclerosis. Brain 1997;120:1865–1876.
Koepp MJ, Richardson MP, Labbe C, Brooks DJ, Cunningham VJ, Ashburner J, et al. 11C-flumazenil PET, volumetric MRI, and quantitative pathology in mesial temporal lobe epilepsy. Neurology 1997;49:764–773.
Debets RM, Sadzot B, van Isselt JW, Brekelmans GJ, Meiners LC, van Huffelen AO, et al. Is 11C-flumazenil PET superior to 18FDG PET and 123I-iomazenil SPECT in presurgical evaluation of temporal lobe epilepsy? J Neurol Neurosurg Psychiatry 1997;62:141–150.
Meencke HJ, Veith G. Hippocampal sclerosis in epilepsy. In: Luders H, editor. Epilepsy surgery. New York: Raven Press; 1991. p. 705–715.
Araujo D, Santos AC, Velasco TR, Wichert-Ana L, Terra-Bustamanate VC, Alexandre V Jr, et al. Volumetric evidence of bilateral damage in unilateral mesial temporal lobe epilepsy. Epilepsia 2006;47:1354–1359.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ohta, Y., Nariai, T., Ishii, K. et al. Voxel- and ROI-based statistical analyses of PET parameters for guidance in the surgical treatment of intractable mesial temporal lobe epilepsy. Ann Nucl Med 22, 495–503 (2008). https://doi.org/10.1007/s12149-008-0140-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-008-0140-5