Abstract
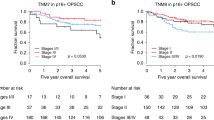
This study addresses the hypothesis that IL-6/STAT3 signaling is of clinical relevance in oropharyngeal squamous cell carcinoma (OPSCC). We evaluated relationships between key components of this pathway in tumors from a unique cohort of n = 59 fully annotated, treatment-naïve patients with OPSCC. The multiplex Opal platform was utilized for immunofluorescence (IF) analysis of tissues to detect IL-6 and phosphorylated STAT3 (pSTAT3), taking into consideration its nuclear versus cytoplasmic localization. Abundant staining for both IL-6 and pSTAT3 was evident in tumor-rich regions of each specimen. IL-6 correlated with cytoplasmic pSTAT3 but not nuclear or total pSTAT3 in this cohort of OPSCC tumors, regardless of p16 status (r = 0.682, p < 0.0001). There was a significant association between increased total pSTAT3, nuclear pSTAT3, cytoplasmic pSTAT3 and IL-6 in p16 negative tumors. Our data indicate STAT3 phosphorylation was a key feature in p16-negative OPSCC tumors. When IL-6 data was stratified by median expression in tumors, there was no association with overall survival. In contrast, both total and nuclear pSTAT3 were significant predictors of poor overall and disease free survival. This strong inverse relationship with overall survival was present in p16 negative tumors for both total and nuclear pSTAT3, but not in p16 positive OPSCC tumors. Together these data indicate that activation of the STAT3 signaling pathway is a marker of p16 negative tumors and relevant to OPSCC prognosis and a potential target for treatment of this more aggressive OPSCC sub-population.



Similar content being viewed by others
References
Pignon JP, et al. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92(1):4–14.
Clarke P, et al. Speech and swallow rehabilitation in head and neck cancer: United Kingdom National Multidisciplinary Guidelines. J Laryngol Otol. 2016;130(S2):S176–80.
Ang KK, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35.
Qian G, et al. Heregulin and HER3 are prognostic biomarkers in oropharyngeal squamous cell carcinoma. Cancer. 2015;121(20):3600–11.
Wang D, et al. HER3 targeting sensitizes HNSCC to cetuximab by reducing HER3 activity and HER2/HER3 dimerization: evidence from cell line and patient-derived xenograft models. Clin Cancer Res. 2017;23(3):677–86.
Fayette J, et al. Randomized phase II study of duligotuzumab (MEHD7945A) vs. cetuximab in squamous cell carcinoma of the head and neck (MEHGAN Study). Front Oncol. 2016;6:232.
Saba NF. Commentary: randomized phase II study of duligotuzumab (MEHD7945A) vs. cetuximab in squamous cell carcinoma of the head and neck (MEHGAN Study). Front Oncol. 2017;7:31.
Steuer CE, et al. A correlative analysis of PD-L1, PD-1, PD-L2, EGFR, HER2, and HER3 expression in oropharyngeal squamous cell carcinoma. Mol Cancer Ther. 2018;17(3):710–6.
Gao J, Zhao S, Halstensen TS. Increased interleukin-6 expression is associated with poor prognosis and acquired cisplatin resistance in head and neck squamous cell carcinoma. Oncol Rep. 2016;35(6):3265–74.
Leon X, et al. Expression of IL-1alpha correlates with distant metastasis in patients with head and neck squamous cell carcinoma. Oncotarget 2015;6(35):37398–409.
Guenin S, et al. Interleukin-32 expression is associated with a poorer prognosis in head and neck squamous cell carcinoma. Mol Carcinog. 2014;53(8):667–73.
Green VL, et al. Serum IL10, IL12 and circulating CD4+ CD25 high T regulatory cells in relation to long-term clinical outcome in head and neck squamous cell carcinoma patients. Int J Oncol. 2012;40(3):833–9.
Hathaway B, et al. Multiplexed analysis of serum cytokines as biomarkers in squamous cell carcinoma of the head and neck patients. Laryngoscope. 2005;115(3):522–7.
Rose-John S, et al. Interleukin-6 biology is coordinated by membrane-bound and soluble receptors: role in inflammation and cancer. J Leukoc Biol. 2006;80(2):227–36.
Fisher DT, Appenheimer MM, Evans SS. The two faces of IL-6 in the tumor microenvironment. Semin Immunol. 2014;26(1):38–47.
Scheller J, et al. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813(5):878–88.
Johnson DE, O’Keefe RA, Grandis JR. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol. 2018;15(4):234–48.
Dominguez C, David JM, Palena C. Epithelial-mesenchymal transition and inflammation at the site of the primary tumor. Semin Cancer Biol. 2017;47:177–84.
Kiesel BF, et al. Toxicity, pharmacokinetics and metabolism of a novel inhibitor of IL-6-induced STAT3 activation. Cancer Chemother Pharmacol. 2016;78(6):1225–35.
Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–74.
Grandis JR, et al. Constitutive activation of Stat3 signaling abrogates apoptosis in squamous cell carcinogenesis in vivo. Proc Natl Acad Sci USA. 2000;97(8):4227–32.
Gaykalova DA, et al. NF-kappaB and stat3 transcription factor signatures differentiate HPV-positive and HPV-negative head and neck squamous cell carcinoma. Int J Cancer. 2015;137(8):1879–89.
Colomiere M, et al. Cross talk of signals between EGFR and IL-6R through JAK2/STAT3 mediate epithelial-mesenchymal transition in ovarian carcinomas. Br J Cancer. 2009;100(1):134–44.
Squarize CH, et al. Molecular cross-talk between the NFkappaB and STAT3 signaling pathways in head and neck squamous cell carcinoma. Neoplasia 2006;8(9):733–46.
Verma G, et al. Characterization of key transcription factors as molecular signatures of HPV-positive and HPV-negative oral cancers. Cancer Med. 2017;6(3):591–604.
Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9(11):798–809.
Zhang Y, et al. Activation of Stat3 in v-Src-transformed fibroblasts requires cooperation of Jak1 kinase activity. J Biol Chem. 2000;275(32):24935–44.
Bharti R, Dey G, Mandal M. Cancer development, chemoresistance, epithelial to mesenchymal transition and stem cells: a snapshot of IL-6 mediated involvement. Cancer Lett. 2016;375(1):51–61.
Wendt MK, et al. STAT3 and epithelial-mesenchymal transitions in carcinomas. JAKSTAT. 2014;3(1):e28975.
Russo N, et al. Cytokines in saliva increase in head and neck cancer patients after treatment. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122(4):483–90 e1.
Zang C, et al. IL-6/STAT3/TWIST inhibition reverses ionizing radiation-induced EMT and radioresistance in esophageal squamous carcinoma. Oncotarget. 2017;8(7):11228–38.
Yadav A, et al. Bazedoxifene enhances the anti-tumor effects of cisplatin and radiation treatment by blocking IL-6 signaling in head and neck cancer. Oncotarget. 2017;8(40):66912–24.
Centurione L, Aiello FB. DNA Repair and Cytokines: TGF-beta, IL-6, and Thrombopoietin as Different Biomarkers of Radioresistance. Front Oncol. 2016;6:175.
Chen Y, et al. IL-6 signaling promotes DNA repair and prevents apoptosis in CD133+ stem-like cells of lung cancer after radiation. Radiat Oncol. 2015;10:227.
Tamatani T, et al. Enhanced radiosensitization and chemosensitization in NF-kappaB-suppressed human oral cancer cells via the inhibition of gamma-irradiation- and 5-FU-induced production of IL-6 and IL-8. Int J Cancer. 2004;108(6):912–21.
Stanam A, et al. Upregulated interleukin-6 expression contributes to erlotinib resistance in head and neck squamous cell carcinoma. Mol Oncol. 2015;9(7):1371–83.
Matsuoka Y, et al. IL-6 controls resistance to radiation by suppressing oxidative stress via the Nrf2-antioxidant pathway in oral squamous cell carcinoma. Br J Cancer. 2016;115(10):1234–44.
Mace TA, et al. IL-6 and PD-L1 antibody blockade combination therapy reduces tumour progression in murine models of pancreatic cancer. Gut. 2018;67(2):320–32.
Acknowledgements
Research reported in this publication was supported in part by the Biostatistics and Bioinformatics Shared Resource of Winship Cancer Institute of Emory University and NIH/NCI under Award Numbers P30CA138292 and 1R01CA208253-01 (G. Lesinski).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest related to this work.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lesinski, G.B., Nannapaneni, S., Griffith, C.C. et al. Interleukin-6/STAT3 Signaling is Prominent and Associated with Reduced Overall Survival in p16 Negative Oropharyngeal Squamous Cell Carcinoma. Head and Neck Pathol 13, 304–312 (2019). https://doi.org/10.1007/s12105-018-0962-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-018-0962-y