Abstract
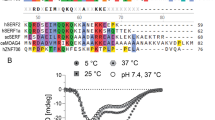
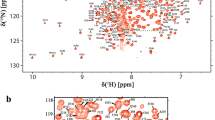
A 25-residue elongation at the N-terminus endows parvulin 17 (Par17) with altered functional properties compared to parvulin 14 (Par14), such as an enhanced influence on microtubule assembly. Therefore the three-dimensional structure of this N-terminal elongation is of particular interest. Here, we report the nearly complete 1H, 13C and 15N chemical shift assignments of Par17. Subsequent chemical shift index analysis indicated that Par17 features a parvulin-type PPIase domain at the C-terminus, analogous to Par14, and an unstructured N-terminus encompassing the first 60 residues. Hence the N-terminus of Par17 apparently adopts a functionally-relevant structure only in presence of the respective interaction partner(s).


Similar content being viewed by others
References
Fanghänel J, Fischer G (2004) Insights into the catalytic mechanism of peptidyl prolyl cis/trans isomerases. Front Biosci 9:3453–3478
Kessler D, Papatheodorou P, Stratmann T, Dian EA, Hartmann-Fatu C, Rassow J, Bayer P, Mueller JW (2007) The DNA binding parvulin Par17 is targeted to the mitochondrial matrix by a recently evolved prepeptide uniquely present in Hominidae. BMC Biol 5:37
Lu PJ, Zhou XZ, Shen M, Lu KP (1999) Function of WW domains as phosphoserine- or phosphothreonine-binding modules. Science 283:1325–1328
Mueller JW, Kessler D, Neumann D, Stratmann T, Papatheodorou P, Hartmann-Fatu C, Bayer P (2006) Characterization of novel elongated parvulin isoforms that are ubiquitously expressed in human tissues and originate from alternative transcription initiation. BMC Mol Biol 7:9
Mueller JW, Link NM, Matena A, Hoppstock L, Rüppel A, Bayer P, Blankenfeldt W (2011) Crystallographic proof for an extended hydrogen-bonding network in small prolyl isomerases. J Am Chem Soc 133:20096–20099
Reimer T, Weiwad M, Schierhorn A, Ruecknagel PK, Rahfeld JU, Bayer P, Fischer G (2003) Phosphorylation of the N-terminal domain regulates subcellular localization and DNA binding properties of the peptidyl-prolyl cis/trans isomerase hPar14. J Mol Biol 330:955–966
Schubert M, Labudde D, Oschkinat H, Schmieder P (2002) A software tool for the prediction of Xaa–Pro peptide bond conformations in proteins based on 13C chemical shift statistics. J Biomol NMR 24:149–154
Sekerina E, Rahfeld JU, Müller J, Fanghänel J, Rascher C, Fischer G, Bayer P (2000) NMR solution structure of hPar14 reveals similarity to the peptidyl prolyl cis/trans isomerase domain of the mitotic regulator hPin1 but indicates a different functionality of the protein. J Mol Biol 301:1003–1017
Surmacz TA, Bayer E, Rahfeld JU, Fischer G, Bayer P (2002) The N-terminal basic domain of human parvulin hPar14 is responsible for the entry to the nucleus and high-affinity DNA-binding. J Mol Biol 321:235–247
Terada T, Shirouzu M, Fukumori Y, Fujimori F, Ito Y, Kigawa T, Yokoyama S, Uchida T (2001) Solution structure of the human parvulin-like peptidyl prolyl cis/trans isomerase, hPar14. J Mol Biol 305:917–926
Thiele A, Krentzlin K, Erdmann F, Rauh D, Hause G, Zerweck J, Kilka S, Pösel S, Fischer G, Schutkowski M, Weiwad M (2011) Parvulin 17 promotes microtubule assembly by its peptidyl-prolyl cis/trans isomerase activity. J Mol Biol 411:896–909
Uchida T, Fujimori F, Tradler T, Fischer G, Rahfeld JU (1999) Identification and characterization of a 14 kDa human protein as a novel parvulin-like peptidyl prolyl cis/trans isomerase. FEBS Lett 446:278–282
Wishart DS, Sykes BD (1994) The 13C chemical-shift index: a simple method for the identification of protein secondary structure using 13C chemical-shift data. J Biomol NMR 4:171–180
Wishart DS, Bigam CG, Yao J, Abildgaard F, Dyson HJ, Oldfield E, Markley JL, Sykes BD (1995) 1H, 13C and 15N chemical shift referencing in biomolecular NMR. J Biomol NMR 6:135–140
Yaffe MB, Schutkowski M, Shen M, Zhou XZ, Stukenberg PT, Rahfeld JU, Xu J, Kuang J, Kirschner MW, Fischer G, Cantley LC, Lu KP (1997) Sequence-specific and phosphorylation-dependent proline isomerization: a potential mitotic regulatory mechanism. Science 278:1957–1960
Zoldák G, Aumüller T, Lücke C, Hritz J, Oostenbrink C, Fischer G, Schmid FX (2009) A library of fluorescent peptides for exploring the substrate specificities of prolyl isomerases. Biochemistry 48:10423–10436
Acknowledgments
The authors are grateful to Prof. Jochen Balbach (University of Halle, Germany) for providing access to the 800 MHz NMR spectrometer. This work was supported by German Ministry for Research and Education Grants BMBF 0315638B and 03IS2211H.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lin, YJ., Schmidt, A., Burgardt, N.I. et al. 1H, 13C and 15N resonance assignments of human parvulin 17. Biomol NMR Assign 7, 325–329 (2013). https://doi.org/10.1007/s12104-012-9438-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12104-012-9438-2