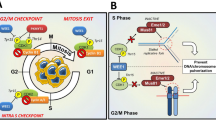
Abstract
Due to the bottlenecks encountered in traditional treatment for tumor, more effective drug targets need to be developed. Cell division cycle 7 kinase plays an important role in DNA replication, DNA repair and recombination signaling pathways. In this review, we first describe recent studies on the role of CDC7 in DNA replication in normal human tissues, and then we integrate new evidence focusing on the important role of CDC7 in replication stress tolerance of tumor cells and its impact on the prognosis of clinical oncology patients. Finally, we comb through the CDC7 inhibitors identified in recent studies as a reference for further research in clinical practice.



Similar content being viewed by others
Abbreviations
- CDC7:
-
Cell division cycle 7
- OS:
-
Overall survival
- PD-1:
-
Programmed cell death 1
- PD-L1:
-
Programmed cell death 1 ligand 1
- EGFR:
-
Epidermal growth factor receptor
- CDK9:
-
Cyclin-dependent kinase 9
- DFS:
-
Disease-free survival
- IRAK1:
-
Interleukin-1 receptor-associated kinase 1
- MCM2:
-
Minichromosome maintenance complex component 2
- ASK:
-
DBF4 zinc finger
- MCM:
-
Minichromosome maintenance protein complex
- CDC45:
-
Cell division cycle 45
- CDK:
-
Cyclin-dependent kinase
- MRE11:
-
Double-strand break repair protein MRE11A
- ATR:
-
ATR serine/threonine kinase
- CHK1:
-
Serine/threonine-protein kinase chk1
- RIF1:
-
Replication timing regulatory factor 1
- PP1:
-
Protein phosphatase 1
- CK1γ1:
-
Casein kinase 1 gamma 1
- MYB:
-
MYB Proto-oncogene, transcription factor
- PGK1:
-
Protein kinase CGMP-dependent 1
- MYC:
-
MYC proto-oncogene, BHLH transcription factor
- CCND1:
-
Cyclin d1
- RAD54L:
-
DNA repair and recombination protein RAD54-like
References
Cheng Z, Wei-Qi J, Jin D. New insights on sorafenib resistance in liver cancer with correlation of individualized therapy. Biochim Biophys Acta Rev Cancer. 2020. https://doi.org/10.1016/j.bbcan.2020.188382.
Vogel A, Qin S, Kudo M, Su Y, Hudgens S, Yamashita T, Yoon JH, Fartoux L, Simon K, Lopez C, Sung M, Mody K, Ohtsuka T, Tamai T, Bennett L, Meier G, Breder V. Lenvatinib versus sorafenib for first-line treatment of unresectable hepatocellular carcinoma: patient-reported outcomes from a randomised, open-label, non-inferiority, phase 3 trial. Lancet Gastroenterol Hepatol. 2021. https://doi.org/10.1016/S2468-1253(21)00110-2.
Liu D, Gao S, Zhai Y, Yang X, Zhai G. Research progress of tumor targeted drug delivery based on PD-1/PD-L1. Int J Pharm. 2022. https://doi.org/10.1016/j.ijpharm.2022.121527.
Yamada M, Masai H, Bartek J. Regulation and roles of Cdc7 kinase under replication stress. Cell Cycle. 2014. https://doi.org/10.4161/cc.29251.
Masai H. A novel p53-Cdc7 link induced by genotoxic stress. Cell Cycle. 2017. https://doi.org/10.1080/15384101.2017.1304746.
Liang XL, Wang YL, Wang PR. MiR-200a with CDC7 as a direct target declines cell viability and promotes cell apoptosis in Wilm’s tumor via Wnt/beta-catenin signaling pathway. Mol Cell Biochem. 2021. https://doi.org/10.1007/s11010-021-04090-9.
Cao JX, Lu Y. Targeting CDC7 improves sensitivity to chemotherapy of esophageal squamous cell carcinoma. Onco Targets Ther. 2019. https://doi.org/10.2147/OTT.S183629.
Gad SA, Ali HEA, Gaballa R, Abdelsalam RM, Zerfaoui M, Ali HI, Salama SH, Kenawy SA, Kandil E, Abd EZY. Targeting CDC7 sensitizes resistance melanoma cells to BRAF(V600E)-specific inhibitor by blocking the CDC7/MCM2-7 pathway. Sci Rep. 2019. https://doi.org/10.1038/s41598-019-50732-w.
Li Q, Xie W, Wang N, Li C, Wang M. CDC7-dependent transcriptional regulation of RAD54L is essential for tumorigenicity and radio-resistance of glioblastoma. Transl Oncol. 2018. https://doi.org/10.1016/j.tranon.2018.01.003.
Mclaughlin RP, He J, van der Noord VE, Redel J, Foekens JA, Martens JWM, Smid M, Zhang Y, van de Water B. A kinase inhibitor screen identifies a dual cdc7/CDK9 inhibitor to sensitise triple-negative breast cancer to EGFR-targeted therapy. Breast Cancer Res. 2019. https://doi.org/10.1186/s13058-019-1161-9.
Zhuang L, Yang Z, Meng Z. Upregulation of BUB1B, CCNB1, CDC7, CDC20, and MCM3 in tumor tissues predicted worse overall survival and disease-free survival in hepatocellular carcinoma patients. Biomed Res Int. 2018. https://doi.org/10.1155/2018/7897346.
Wang C, Vegna S, Jin H, Benedict B, Lieftink C, Ramirez C, de Oliveira RL, Morris B, Gadiot J, Wang W, du Chatinier A, Wang L, Gao D, Evers B, Jin G, Xue Z, Schepers A, Jochems F, Sanchez AM, Mainardi S, Te Riele H, Beijersbergen RL, Qin W, Akkari L, Bernards R. Inducing and exploiting vulnerabilities for the treatment of liver cancer. Nature. 2019. https://doi.org/10.1038/s41586-019-1607-3.
Guo Y, Wang J, Benedict B, Yang C, van Gemert F, Ma X, Gao D, Wang H, Zhang S, Lieftink C, Beijersbergen RL, Te Riele H, Qiao X, Gao Q, Sun C, Qin W, Bernards R, Wang C. Targeting CDC7 potentiates ATR-CHK1 signaling inhibition through induction of DNA replication stress in liver cancer. Genome Med. 2021. https://doi.org/10.1186/s13073-021-00981-0.
Huggett MT, Tudzarova S, Proctor I, Loddo M, Keane MG, Stoeber K, Williams GH, Pereira SP. Cdc7 is a potent anti-cancer target in pancreatic cancer due to abrogation of the DNA origin activation checkpoint. Oncotarget. 2016. https://doi.org/10.18632/oncotarget.7611.
Jaafari-Ashkavandi Z, Ashraf MJ, Abbaspoorfard AA. Overexpression of CDC7 in malignant salivary gland tumors correlates with tumor differentiation. Braz J Otorhinolaryngol. 2019. https://doi.org/10.1016/j.bjorl.2017.11.004.
Jin S, Ma H, Yang W, Ju H, Wang L, Zhang Z. Cell division cycle 7 is a potential therapeutic target in oral squamous cell carcinoma and is regulated by E2F1. J Mol Med (Berl). 2018. https://doi.org/10.1007/s00109-018-1636-7.
Zografos E, Anagnostopoulos AK, Papadopoulou A, Legaki E, Zagouri F, Marinos E, Tsangaris GT, Gazouli M. Serum proteomic signatures of male breast cancer. Cancer Genomics Proteom. 2019. https://doi.org/10.21873/cgp.20118.
Forest F, Laville D, Da Cruz V, Casteillo F, Clemenson A, Yvorel V, Picot T. WHO grading system for invasive pulmonary lung adenocarcinoma reveals distinct molecular signature: an analysis from the cancer genome atlas database. Exp Mol Pathol. 2022. https://doi.org/10.1016/j.yexmp.2022.104756.
Wang Y, Wang Y, Duan X, Wang Y, Zhang Z. Interleukin-1 receptor-associated kinase 1 correlates with metastasis and invasion in endometrial carcinoma. J Cell Biochem. 2018. https://doi.org/10.1002/jcb.26416.
Ma Q, Zhang J, Zhang M, Lan H, Yang Q, Li C, Zeng L. MicroRNA-29b targeting of cell division cycle 7-related protein kinase (CDC7) regulated vascular smooth muscle cell (VSMC) proliferation and migration. Ann Transl Med. 2020. https://doi.org/10.21037/atm-20-6856.
Rainey MD, Quinlan A, Cazzaniga C, Mijic S, Martella O, Krietsch J, Goder A, Lopes M, Santocanale C. CDC7 kinase promotes MRE11 fork processing, modulating fork speed and chromosomal breakage. EMBO Rep. 2020. https://doi.org/10.15252/embr.201948920.
Dick SD, Federico S, Hughes SM, Pye VE, O’Reilly N, Cherepanov P. Structural basis for the activation and target site specificity of CDC7 kinase. Structure. 2020. https://doi.org/10.1016/j.str.2020.05.010.
Labib K. How do Cdc7 and cyclin-dependent kinases trigger the initiation of chromosome replication in eukaryotic cells? Genes Dev. 2010. https://doi.org/10.1101/gad.1933010.
Moiseeva T, Hood B, Schamus S, O’Connor MJ, Conrads TP, Bakkenist CJ. ATR kinase inhibition induces unscheduled origin firing through a Cdc7-dependent association between GINS and And-1. Nat Commun. 2017. https://doi.org/10.1038/s41467-017-01401-x.
Moiseeva TN, Yin Y, Calderon MJ, Qian C, Schamus-Haynes S, Sugitani N, Osmanbeyoglu HU, Rothenberg E, Watkins SC, Bakkenist CJ. An ATR and CHK1 kinase signaling mechanism that limits origin firing during unperturbed DNA replication. Proc Natl Acad Sci U S A. 2019. https://doi.org/10.1073/pnas.1903418116.
Rainey MD, Bennett D, O’Dea R, Zanchetta ME, Voisin M, Seoighe C, Santocanale C. ATR restrains DNA synthesis and mitotic catastrophe in response to CDC7 inhibition. Cell Rep. 2020. https://doi.org/10.1016/j.celrep.2020.108096.
Li Z, Xu X. Post-Translational modifications of the mini-chromosome maintenance proteins in DNA replication. Genes (Basel). 2019. https://doi.org/10.3390/genes10050331.
Ciccia A, Elledge SJ. The DNA damage response: making it safe to play with knives. Mol Cell. 2010. https://doi.org/10.1016/j.molcel.2010.09.019.
Bian R, Dang W, Song X, Liu L, Jiang C, Yang Y, Li Y, Li L, Li X, Hu Y, Bao R, Liu Y. Rac GTPase activating protein 1 promotes gallbladder cancer via binding DNA ligase 3 to reduce apoptosis. Int J Biol Sci. 2021. https://doi.org/10.7150/ijbs.58857.
Yang CC, Suzuki M, Yamakawa S, Uno S, Ishii A, Yamazaki S, Fukatsu R, Fujisawa R, Sakimura K, Tsurimoto T, Masai H. Claspin recruits Cdc7 kinase for initiation of DNA replication in human cells. Nat Commun. 2016. https://doi.org/10.1038/ncomms12135.
Yang CC, Kato H, Shindo M, Masai H. Cdc7 activates replication checkpoint by phosphorylating the Chk1-binding domain of Claspin in human cells. Elife. 2019. https://doi.org/10.7554/eLife.50796.
Lin YL, Pasero P. Replication stress: from chromatin to immunity and beyond. Curr Opin Genet Dev. 2021. https://doi.org/10.1016/j.gde.2021.08.004.
Gaillard H, Garcia-Muse T, Aguilera A. Replication stress and cancer. Nat Rev Cancer. 2015. https://doi.org/10.1038/nrc3916.
Datta A, Ghatak D, Das S, Banerjee T, Paul A, Butti R, Gorain M, Ghuwalewala S, Roychowdhury A, Alam SK, Das P, Chatterjee R, Dasgupta M, Panda CK, Kundu GC, Roychoudhury S. p53 gain-of-function mutations increase Cdc7-dependent replication initiation. EMBO Rep. 2017. https://doi.org/10.15252/embr.201643347.
Li X, Qian X, Jiang H, Xia Y, Zheng Y, Li J, Huang BJ, Fang J, Qian CN, Jiang T, Zeng YX, Lu Z. Nuclear PGK1 alleviates ADP-dependent inhibition of CDC7 to promote DNA replication. Mol Cell. 2018. https://doi.org/10.1016/j.molcel.2018.09.007.
Hills SA, Diffley JF. DNA replication and oncogene-induced replicative stress. Curr Biol. 2014. https://doi.org/10.1016/j.cub.2014.04.012.
Sugiyama T, Chen Y. Biochemical reconstitution of UV-induced mutational processes. Nucleic Acids Res. 2019. https://doi.org/10.1093/nar/gkz335.
Tartarone A, Roviello G, Lerose R, Roudi R, Aieta M, Zoppoli P. Anti-PD-1 versus anti-PD-L1 therapy in patients with pretreated advanced non-small-cell lung cancer: a meta-analysis. Future Oncol. 2019. https://doi.org/10.2217/fon-2018-0868.
Petrelli F, Ferrara R, Signorelli D, Ghidini A, Proto C, Roudi R, Sabet MN, Facelli S, Garassino MC, Luciani A, Roviello G. Immune checkpoint inhibitors and chemotherapy in first-line NSCLC: a meta-analysis. Immunotherapy. 2021. https://doi.org/10.2217/imt-2020-0224.
Sato H, Niimi A, Yasuhara T, Permata TBM, Hagiwara Y, Isono M, Nuryadi E, Sekine R, Oike T, Kakoti S, Yoshimoto Y, Held KD, Suzuki Y, Kono K, Miyagawa K, Nakano T, Shibata A. DNA double-strand break repair pathway regulates PD-L1 expression in cancer cells. Nat Commun. 2017. https://doi.org/10.1038/s41467-017-01883-9.
Irie T, Asami T, Sawa A, Uno Y, Hanada M, Taniyama C, Funakoshi Y, Masai H, Sawa M. Discovery of novel furanone derivatives as potent Cdc7 kinase inhibitors. Eur J Med Chem. 2017. https://doi.org/10.1016/j.ejmech.2017.02.030.
Irie T, Asami T, Sawa A, Uno Y, Taniyama C, Funakoshi Y, Masai H, Sawa M. Discovery of AS-0141, a potent and selective inhibitor of CDC7 kinase for the treatment of solid cancers. J Med Chem. 2021. https://doi.org/10.1021/acs.jmedchem.1c01319.
Iwai K, Nambu T, Kashima Y, Yu J, Eng K, Miyamoto K, Kakoi K, Gotou M, Takeuchi T, Kogame A, Sappal J, Murai S, Haeno H, Kageyama SI, Kurasawa O, Niu H, Kannan K, Ohashi A. A CDC7 inhibitor sensitizes DNA-damaging chemotherapies by suppressing homologous recombination repair to delay DNA damage recovery. Sci Adv. 2021. https://doi.org/10.1126/sciadv.abf0197.
Iwai K, Nambu T, Dairiki R, Ohori M, Yu J, Burke K, Gotou M, Yamamoto Y, Ebara S, Shibata S, Hibino R, Nishizawa S, Miyazaki T, Homma M, Oguro Y, Imada T, Cho N, Uchiyama N, Kogame A, Takeuchi T, Kurasawa O, Yamanaka K, Niu H, Ohashi A. Molecular mechanism and potential target indication of TAK-931, a novel CDC7-selective inhibitor. Sci Adv. 2019. https://doi.org/10.1126/sciadv.aav3660.
Zhou X, Ouerdani A, Diderichsen PM, Gupta N. Population pharmacokinetics of TAK-931, a cell division cycle 7 kinase inhibitor, in patients with advanced solid tumors. J Clin Pharmacol. 2021. https://doi.org/10.1002/jcph.1974.
Sasi NK, Tiwari K, Soon FF, Bonte D, Wang T, Melcher K, Xu HE, Weinreich M. The potent Cdc7-Dbf4 (DDK) kinase inhibitor XL413 has limited activity in many cancer cell lines and discovery of potential new DDK inhibitor scaffolds. PLoS ONE. 2014. https://doi.org/10.1371/journal.pone.0113300.
Koltun ES, Tsuhako AL, Brown DS, Aay N, Arcalas A, Chan V, Du H, Engst S, Ferguson K, Franzini M, Galan A, Holst CR, Huang P, Kane B, Kim MH, Li J, Markby D, Mohan M, Noson K, Plonowski A, Richards SJ, Robertson S, Shaw K, Stott G, Stout TJ, Young J, Yu P, Zaharia CA, Zhang W, Zhou P, Nuss JM, Xu W, Kearney PC. Discovery of XL413, a potent and selective CDC7 inhibitor. Bioorg Med Chem Lett. 2012. https://doi.org/10.1016/j.bmcl.2012.04.024.
Erbayraktar Z, Alural B, Erbayraktar RS, Erkan EP. Cell division cycle 7-kinase inhibitor PHA-767491 hydrochloride suppresses glioblastoma growth and invasiveness. Cancer Cell Int. 2016. https://doi.org/10.1186/s12935-016-0364-8.
Chen EW, Tay NQ, Brzostek J, Gascoigne NRJ, Rybakin V. A dual inhibitor of Cdc7/Cdk9 potently suppresses T cell activation. Front Immunol. 2019. https://doi.org/10.3389/fimmu.2019.01718.
Sawa M, Masai H. Drug design with Cdc7 kinase: a potential novel cancer therapy target. Drug Des Devel Ther. 2009. https://doi.org/10.2147/dddt.s4303.
Acknowledgements
This work was supported by the Scientific and Technological Innovation Major Base of Guangxi (No. 2018-15-Z04), Guangxi Key Research and Development Project (No. AB20117001), Guangxi science and technology bases and talent special project (No. AD17129062).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Ethical approval
This study did not involve human participants and/or animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, R., Huang, Y. CDC7 as a novel biomarker and druggable target in cancer. Clin Transl Oncol 24, 1856–1864 (2022). https://doi.org/10.1007/s12094-022-02853-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-022-02853-4