Abstract
Background
Antibiotic use at the time of chemotherapy has been linked with inferior outcomes among a number of solid tumors. The current study aims at further assessing this observation among metastatic colorectal cancer patients treated with first-line systemic chemotherapy.
Methods
This is a pooled analysis of three clinical trial datasets (NCT00384176; NCT00272051; NCT00305188) that were accessed from the Project Data Sphere platform. Kaplan–Meier survival estimates were used to evaluate the impact of antibiotic use on overall and progression-free survival and multivariable Cox regression models were employed to further assess this impact.
Results
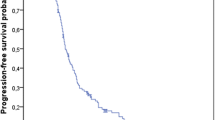
A total of 1446 patients were included in the current analysis. These include 108 patients who received antibiotics before the start of chemotherapy, 499 patients who received antibiotics after the start of chemotherapy, and 839 patients who did not receive antibiotics. Using Kaplan–Meier survival estimates, the use of antibiotics prior to the start of chemotherapy was associated with worse progression-free (P = 0.001) and overall survival (P < 0.001). Likewise, when multivariable Cox regression analyses were conducted, prior antibiotic use is associated with worse progression-free (HR for antibiotic use during chemotherapy versus antibiotic use prior to chemotherapy = 0.764; 95% CI 0.604–0.966; P = 0.024) and overall survival (HR for antibiotic use during chemotherapy versus antibiotic use prior to chemotherapy = 0.710; 95% CI 0.537–0.940; P = 0.017).
Conclusion
Antibiotic use before (but not following) the start of 5FU-based chemotherapy is associated with worse progression-free and overall survival among patients with metastatic colorectal cancer.


Similar content being viewed by others
Data availability
The data that support the findings of this study are openly available in https://www.projectdatasphere.org.
References
Kim JH. Chemotherapy for colorectal cancer in the elderly. World J Gastroenterol. 2015;21(17):5158–66.
Millan M, Merino S, Caro A, Feliu F, Escuder J, Francesch T. Treatment of colorectal cancer in the elderly. World J Gastrointest Oncol. 2015;7(10):204–20.
Abdel-Rahman O. Prognostic value of baseline ALBI score among patients with colorectal liver metastases: a pooled analysis of two randomized trials. Clin Colorectal Cancer. 2019;18(1):e61–e6868.
Lalani AA, Xie W, Braun DA, Kaymakcalan M, Bosse D, Steinharter JA, et al. Effect of antibiotic use on outcomes with systemic therapies in metastatic renal cell carcinoma. Eur Urol Oncol. 2019. https://doi.org/10.1016/j.euo.2019.09.001.
Pinato DJ, Howlett S, Ottaviani D, Urus H, Patel A, Mineo T, et al. Association of prior antibiotic treatment with survival and response to immune checkpoint inhibitor therapy in patients with cancer. JAMA Oncol. 2019;5(12):1774–8.
Elkrief A, Derosa L, Kroemer G, Zitvogel L, Routy B. The negative impact of antibiotics on outcomes in cancer patients treated with immunotherapy: a new independent prognostic factor? Ann Oncol. 2019;30(10):1572–9.
https://www.projectdatasphere.org/projectdatasphere/html/home. Accessed 10 Jul 2017.
Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, et al. Final results from PRIME: randomized phase III study of panitumumab with FOLFOX4 for first-line treatment of metastatic colorectal cancer. Ann Oncol. 2014;25(7):1346–55.
Hecht JR, Mitchell E, Chidiac T, Scroggin C, Hagenstad C, Spigel D, et al. A randomized phase IIIB trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J Clin Oncol. 2009;27(5):672–80.
Schmoll HJ, Cunningham D, Sobrero A, Karapetis CS, Rougier P, Koski SL, et al. Cediranib with mFOLFOX6 versus bevacizumab with mFOLFOX6 as first-line treatment for patients with advanced colorectal cancer: a double-blind, randomized phase III study (HORIZON III). J Clin Oncol. 2012;30(29):3588–95.
Abdel-Rahman O. Impact of sex on chemotherapy toxicity and efficacy among patients with metastatic colorectal cancer: pooled analysis of 5 randomized trials. Clin Colorectal Cancer. 2019;18(2):110–5.e2.
Abdel-Rahman O. Effect of body mass index on 5-FU-based chemotherapy toxicity and efficacy among patients with metastatic colorectal cancer; a pooled analysis of 5 randomized trials. Clin Colorectal Cancer. 2019;18(4):e385–e393393.
Abdel-Rahman O, Karachiwala H. Impact of age on toxicity and efficacy of 5-FU-based combination chemotherapy among patients with metastatic colorectal cancer; a pooled analysis of five randomized trials. Int J Colorectal Dis. 2019;34(10):1741–7.
Zitvogel L, Galluzzi L, Viaud S, Vétizou M, Daillère R, Merad M, et al. Cancer and the gut microbiota: an unexpected link. Sci Transl Med. 2015;7(271):271ps1-ps1.
Francino MP. Antibiotics and the human gut microbiome: dysbioses and accumulation of resistances. Front Microbiol. 2016;6:1543.
Yuan L, Zhang S, Li H, Yang F, Mushtaq N, Ullah S, et al. The influence of gut microbiota dysbiosis to the efficacy of 5-Fluorouracil treatment on colorectal cancer. Biomed Pharmacother. 2018;108:184–93.
Huemer F, Rinnerthaler G, Westphal T, Hackl H, Hutarew G, Peter Gampenrieder S, et al. Impact of antibiotic treatment on immune-checkpoint blockade efficacy in advanced non-squamous non-small cell lung cancer. Oncotarget. 2018;9(23):16512–20.
Coutzac C, Jouniaux J-M, Paci A, et al. Systemic gut microbial metabolites limit the anti-tumor effect of CTLA-4 blockade in hosts with cancer. Ann Oncol. 2019;30(suppl_5):v1–v24. https://doi.org/10.1093/annonc/mdz238.
Zhang J, Haines C, Watson AJM, Hart AR, Platt MJ, Pardoll DM, et al. Oral antibiotic use and risk of colorectal cancer in the United Kingdom, 1989–2012: a matched case–control study. Gut. 2019;68(11):1971–8.
Zackular JP, Baxter NT, Chen GY, Schloss PD. Manipulation of the gut microbiota reveals role in colon tumorigenesis. mSphere. 2016;1(1):e00001–15.
Wang JL, Chang CH, Lin JW, Wu LC, Chuang LM, Lai MS. Infection, antibiotic therapy and risk of colorectal cancer: a nationwide nested case–control study in patients with Type 2 diabetes mellitus. Int J Cancer. 2014;135(4):956–67.
Acknowledgements
This publication is based on research using information obtained from https://www.projectdatasphere.org, which is maintained by the Project Data Sphere, LLC. Neither Project Data Sphere, LLC nor the owner(s) of any information from the website have contributed to, approved or are in any way responsible for the contents of this publication.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no competing interest.
Ethical approval
Ethical approval was obtained from all participating centers in the three clinical trials included in the current analysis.
Informed consent
Informed consent was obtained from all participants in the three primary clinical trials.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abdel-Rahman, O., Ghosh, S. & Walker, J. Outcomes of metastatic colorectal cancer patients in relationship to prior and concurrent antibiotics use; individual patient data analysis of three clinical trials. Clin Transl Oncol 22, 1651–1656 (2020). https://doi.org/10.1007/s12094-020-02301-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-020-02301-1