Abstract
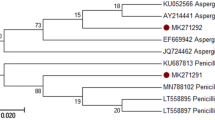
Several fungi and starch-rich industrial residues were screened for itaconic acid (IA) production. Out of 15 strains, only three fungal strains were found to produce IA, which was confirmed by HPLC and GC–MS analysis. These strains were identified as Aspergillus terreus strains C1 and C2, and Ustilago maydis strain C3 by sequencing of 18S rRNA gene and internal transcribed spacer regions. Cis-aconitate decarboxylase (cad) gene, which encodes a key enzyme in IA production in A. terreus, was characterized from strains C1 and C2. C1 and C2 cad gene sequences showed about 96% similarity to the only available GenBank sequence of A. terreus cad gene. 3-D structure and cis-aconitic acid binding pocket of Cad enzyme were predicted by structural modeling. Rice, corn and potato starch wastes were screened for IA production. These materials were enzymatically hydrolyzed under experimentally optimized conditions resulting in the highest glucose production of 230 mg/mL from 20% potato waste. On comparing the production potential of selected strains with different wastes, the best IA production was achieved with strain C1 (255.7 mg/L) using potato waste. Elemental composition as well as batch-to-batch variation in waste substrates were analyzed. The difference in IA production from two different batches of potato waste was found to inversely correlate with their phosphorus content, which indicated that A. terreus produced IA under phosphate limiting condition. The potato waste hydrolysate was deionized to remove inhibitory ions like phosphate, resulting in improved IA production of 4.1 g/L by C1 strain, which is commercially competitive.


Similar content being viewed by others
References
Ferreira JA, Mahboubi A, Lennartsson PR, Taherzadeh MJ (2016) Waste biorefineries using filamentous ascomycetes fungi: present status and future prospects. Bioresour Technol 215:334–345. doi:10.1016/j.biortech.2016.03.018
Songserm P, Thitiprasert S, Tolieng V, Piluk J, Tanasupawat S, Assabumrungrat S, Yang ST, Karnchanatat A, Thongchul N (2015) Regulating pyruvate carboxylase in the living culture of Aspergillus terreus Nrrl 1960 by L-aspartate for enhanced itaconic acid production. Appl Biochem Biotechnol 177:595–609. doi:10.1007/s12010-015-1763-3
El-Imam AA, Kazeem M, Odebisi M, Oke M, Abidoye A (2013) Production of itaconic acid from Jatropha curcas seed cake by Aspergillus terreus. Not Sci Biol 5:57–61. doi:10.15835/nsb518355
El-Imam AA, Chenyu D (2015) Itaconic acid production from sorghum bran: A biorefining approach. J Fundam Renew Energy Appl 5:5. doi:10.4172/2090-4541.S1.002
Dwiarti L, Otsuka M, Miura S, Yaguchi M, Okabe M (2007) Itaconic acid production using sago starch hydrolysate by Aspergillus terreus TN484-M1. Bioresour Technol 98:3329–3337. doi:10.1016/j.biortech.2006.03.016
Kuenz A, Gallenmüller Y, Willke T, Vorlop KD (2012) Microbial production of itaconic acid: developing a stable platform for high product concentrations. Appl Microbiol Biotechnol 96:1209–1216. doi:10.1007/s00253-012-4221-y
Klement T, Milker S, Jager G, Grande P, Dominguez de Maria P, Büchs J (2012) Biomass pretreatment affects Ustilago maydis in producing itaconic acid. Microb Cell Factor 11:43. doi:10.1186/1475-2859-11-43
Bonnarme P, Gillet B, Sepulchre AM, Role C, Beloeil JC, Ducrocq C (1995) Itaconate biosynthesis in Aspergillus terreus. J Bacteriol 177:3573–3578
Aneja KR (2005) Experiments in microbiology plant pathology and biotechnology, 4th edn. New Age International (P) Limited Publishers, New Delhi
Purahong W, Kahl T, Schloter M, Bauhus J, Buscot F, Krüger D (2014) Comparing fungal richness and community composition in coarse woody debris in Central European beech forests under three types of management. Mycol Progress 13:959. doi:10.1007/s11557-013-0954-y
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Irinyi L, Lackner M, de Hoog S, Meyer W (2016) DNA barcoding of fungi causing infections in humans and animals. Fungal Biology 120:125–136. doi:10.1016/j.funbio.2015.04.007
Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJ (2015) The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc 10:845–858. doi:10.1038/nprot.2015.053
Grosdidier A, Zoete V, Michielin O (2011) SwissDock, a protein-small molecule docking web service based on EADock DSS. Nucleic Acids Res 39:W270–W277. doi:10.1093/nar/gkr366
ASTM (2015) Standard test method for determination of total solids in biomass, ASTM Standard E1756-08. American Society for Testing and Materials, International, Philadelphia
ASTM (2003) Standard method for the determination of ash in biomass, ASTM Standard E1755-01. American Society for Testing and Materials, International, Philadelphia
DuBois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356. doi:10.1021/ac60111a017
Olsen SR, Cole CV, Watanabe FS (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate. USDA Circular No. 939. US Government Printing Office, Washington DC
Lohkamp B, Bäuerle B, Rieger PG, Schneider G (2006) Three-dimensional structure of iminodisuccinate epimerase defines the fold of the MmgE/PrpD protein family. J Mol Biol 362:555–566. doi:10.1016/j.jmb.2006.07.051
Apar DK, Turhan M, Zbek BO (2006) Enzymatic hydrolysis of starch by using a sonifier. Chem Eng Commun 193:1117–1126. doi:10.1080/00986440500354424
Okabe M, Lies D, Kanamasa S, Park EY (2009) Biotechnological production of itaconic acid and its biosynthesis in Aspergillus terreus. Appl Microbiol Biotechnol 84:597–606. doi:10.1007/s00253-009-2132-3
Klement T, Büchs J (2013) Itaconic acid: a biotechnological process in change. Bioresour Technol 135:422–431. doi:10.1016/j.biortech.2012.11.141
Schoch CL, Seifert KA, Huhndorf S, Robert V, Spouge JL, Levesque CA, Chen W, Bolchacova E, Voigt K, Crous PW (2012) Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for fungi. PNAS 109:6241–6246. doi:10.1073/pnas.1117018109
Geiser E, Przybilla SK, Friedrich A, Buckel W, Wierckx N, Blank LM, Bolker M (2016) Ustilago maydis produces itaconic acid via the unusual intermediate trans-aconitate. Microb Biotechnol 9:116–126. doi:10.1111/1751-7915.12329
Kanamasa S, Dwiarti L, Okabe M, Park EY (2008) Cloning and functional characterization of the cis-aconitic acid decarboxylase (CAD) gene from Aspergillus terreus. Appl Microbiol Biotechnol 80:223–229. doi:10.1007/s00253-008-1523-1
Tevž G, Benčina M, Legiša M (2010) Enhancing itaconic acid production by Aspergillus terreus. Appl Microbiol Biotechnol 87:1657–1664. doi:10.1007/s00253-010-2642-z
Yahiro K, Takahama T, Park Y, Okabe M (1995) Breeding of Aspergillus terreus mutant TN-484 for itaconic acid production with high yield. J Ferm Bioeng 79:506–508. doi:10.1016/0922-338X(95)91272-7
Riscaldati E, Moresi M, Federici F, Petruccioli M (2000) Effect of pH and stirring rate on itaconate production by Aspergillus terreus. J Biotechnol 83:219–230. doi:10.1016/S0168-1656(00)00322-9
Noda T, Tsuda S, Mori M, Takigawa S, Matsuura-Endo C, Kim SJ, Hashimoto N, Yamauchi H (2006) Determination of the phosphorus content in potato starch using an energy-dispersive X-ray fluorescence method. Food Chem 95:632–637. doi:10.1016/j.foodchem.2005.02.002
Azhar A, Hamdy MK (1979) Sonication effect on potato starch and sweet potato powder. J Food Sci 44:801–804. doi:10.1111/j.1365-2621.1979.tb08505.x
Willke T, Vorlop KD (2001) Biotechnological production of itaconic acid. Appl Microbiol Biotechnol 56:289–295. doi:10.1007/s002530100685
Shah DN, Chattoo BB, Kothari RM, Hegde MV (1993) Starch hydrolysate, an optimal and economical source of carbon for the secretion of citric acid by Yarrowia lipolytica (DS-1). Starch 45:104–109. doi:10.1002/star.19930450308
Acknowledgements
RB thanks the University Grant Commission (UGC), Government of India for award of Senior Research Fellowship (UGC-SRF; reference no. 17-06/2012(i) EU-V). The manuscript represents communication number CSIR-NEERI/KRC/2016/OCT/EBGD/1.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bafana, R., Sivanesan, S. & Pandey, R.A. Itaconic Acid Production by Filamentous Fungi in Starch-Rich Industrial Residues. Indian J Microbiol 57, 322–328 (2017). https://doi.org/10.1007/s12088-017-0661-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12088-017-0661-5