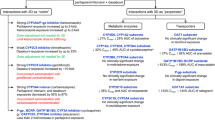
Abstract
Background
The increasing number of direct-acting antiviral (DAA) regimens along with limited number of subjects and co-medications involved in clinical trials results in drug–drug interactions (DDIs) with DAAs is to be determined. We aimed to examine the prevalence and degree of DDIs between DAAs and other co-medications in a territory-wide cohort of chronic hepatitis C virus (HCV) patients.
Methods
DDIs were assigned to three risk categories: Category 1—no clinically significant DDI; category 2—potential clinically significant interaction (monitoring and caution required); category 3—contraindicated (should not be co-administered).
Results
Of 2981 patients (mean age 59.3 ± 12.3 years; male 60.6%), 810 (48.8%) had genotype 1 and 552 (33.2%) genotype 6 HCV among the 1661 patients with HCV genotype tested; 769 (25.8%) received sofosbuvir/velpatasvir, 510 (17.1%) sofosbuvir/ledipasvir, and 865 (29.0%) glecaprevir/pibrentasvir. More than one-fourth (26.3%) of the patients have polypharmacy (≥ 3 co-medications) in all patients, 27.0% in patients received sofosbuvir/velpatasvir, 25.1% in elbasvir/grazoprevir, and 21.2% in glecaprevir/pibrentasvir. 2037 (68.3%) patient experienced DDI (Category 2: 53.1%; Category 3: 15.2%). The commonest drugs leading to DDIs were calcium channel blockers (31.5%) and proton pump inhibitors (23.0%) in category 2; statins (10.2%), antiplatelet/anticoagulants (3.0%) and antipsychotics (2.9%) in category 3. Changing medication was the most common response from physicians in both category 2 and 3 DDIs.
Conclusion
The commonest co-medications leading to contraindication during DAA treatment were statins and antipsychotics. Category 2 and 3 DDIs are often managed by appropriate dose adjustments or temporary discontinuation of relevant co-medications. Careful assessment for potential DDI before DAA use is mandatory to avoid potential harmful effects.
Graphical abstract


Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available due to regulations of the Hospital Authority, but are available from the corresponding author on reasonable request.
Abbreviations
- anti-HCV:
-
HCV antibody
- DAA:
-
Direct-acting antiviral
- DDI:
-
Drug–drug interactions
- HCC:
-
Hepatocellular carcinoma
- HCV:
-
Hepatitis C virus
- IQR:
-
Interquartile range
- PWID:
-
People who inject drugs
- SD:
-
Standard deviation
References
World Health Organization. Global hepatitis report 2017. Website: https://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/. Accessed on 20 Apr 2021.
Polaris Observatory HCVC. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2(3):161–176
Wong GL, Chan HL, Loo CK, et al. Change in treatment paradigm in people who previously injected drugs with chronic hepatitis C in the era of direct-acting antiviral therapy. J Gastroenterol Hepatol. 2019;34(9):1641–1647
Department of Health HG. Hong Kong Viral Hepatitis Action Plan 2020–2024. October 2020. Website: https://www.hepatitis.gov.hk/doc/action_plan/Action%20Plan_Full%20Version_PDF_en.pdf. Accessed on 20 Apr 2021.
Department of Health HG. Surveillance of Viral Hepatitis in Hong Kong – Update Report. Hong Kong; 2019. Website: https://www.chp.gov.hk/files/pdf/viral_hep_sur_report_2019.pdf. Accessed on 30 Sep 2021.
Hui YT, Wong GLH, Fung JYY, et al. Territory-wide population-based study of chronic hepatitis C infection and implications for hepatitis elimination in Hong Kong. Liver Int. 2018;38(11):1911–1919
Mac Nicholas R, Norris S. Review article: optimizing SVR and management of the haematological side effects of peginterferon/ribavirin antiviral therapy for HCV - the role of epoetin, G-CSF and novel agents. Aliment Pharmacol Ther. 2010;31(9):929–937
Banerjee D, Reddy KR. Review article: safety and tolerability of direct-acting anti-viral agents in the new era of hepatitis C therapy. Aliment Pharmacol Ther. 2016;43(6):674–696
Vermehren J, Park JS, Jacobson IM, Zeuzem S. Challenges and perspectives of direct antivirals for the treatment of hepatitis C virus infection. J Hepatol. 2018;69(5):1178–1187
Vermehren J, Peiffer KH, Welsch C, et al. The efficacy and safety of direct acting antiviral treatment and clinical significance of drug-drug interactions in elderly patients with chronic hepatitis C virus infection. Aliment Pharmacol Ther. 2016;44(8):856–865
Kiser JJ, Burton JR Jr, Everson GT. Drug–drug interactions during antiviral therapy for chronic hepatitis C. Nat Rev Gastroenterol Hepatol. 2013;10(10):596–606
Maasoumy B, Port K, Calle Serrano B, et al. The clinical significance of drug–drug interactions in the era of direct-acting anti-viral agents against chronic hepatitis C. Aliment Pharmacol Ther. 2013;38(11–12):1365–1372
Talavera Pons S, Boyer A, Lamblin G, et al. Managing drug-drug interactions with new direct-acting antiviral agents in chronic hepatitis C. Br J Clin Pharmacol. 2017;83(2):269–293
Hui VW, Chan SL, Wong VW, et al. Increasing antiviral treatment uptake improves survival in patients with HBV-related HCC. JHEP Rep. 2020;2(6): 100152
Yip TC, Wong VW, Chan HL, Tse YK, Lui GC, Wong GL. Tenofovir is associated with lower risk of hepatocellular carcinoma than entecavir in patients with chronic HBV infection in China. Gastroenterology. 2020;158(1):215–225 (e6)
Authority H. Hospital authority drug formulary (HADF). Website: https://www.ha.org.hk/hadf/en-us/Updated-HA-Drug-Formulary/Drug-Formulary.html. Accessed on 30 Sep 2021.
Liverpool Uo. Interaction charts for directly acting antivirals and ribavirin. University of Liverpool. Website: https://www.hep-druginteractions.org/checker. Accessed on 30 Sep 2021.
Geddawy A, Ibrahim YF, Elbahie NM, Ibrahim MA. Direct acting anti-hepatitis C virus drugs: clinical pharmacology and future direction. J Transl Int Med. 2017;5(1):8–17
Schulte B, Wubbolding M, Marra F, et al. Frequency of potential drug-drug interactions in the changing field of HCV therapy. Open Forum Infect Dis. 2020;7(2): ofaa040
Hsu PY, Wei YJ, Lee JJ, et al. Comedications and potential drug-drug interactions with direct-acting antivirals in hepatitis C patients on hemodialysis. Clin Mol Hepatol. 2021;27(1):186–196
Mangia A, Scaglione F, Toniutto P, et al. Drug–drug interactions in Italian patients with chronic hepatitis C treated with pangenotypic direct acting agents: insights from a real-world study. Int J Environ Res Public Health. 2021;18(13):7144
Boff da Costa R, Boff Costa M, Longo L, et al. Direct antiviral agents for hepatitis C and drug interaction risk: a retrospective cohort study with real and simulated data on medication interaction, prevalence of comorbidities and comedications. PLoS ONE. 2021;16(2): e0245767
Curry MP, Flamm SL, Milligan S, et al. Prevalence of drug-drug interactions with pangenotypic direct-acting antivirals for hepatitis C and real-world care management in the United States: a retrospective observational study. J Manag Care Spec Pharm. 2021;27(9):1239–1248
Coghlan M, O’Leary A, Melanophy G, Bergin C, Norris S. Pharmacist-led pre-treatment assessment, management and outcomes in a Hepatitis C treatment patient cohort. Int J Clin Pharm. 2019;41(5):1227–1238
Sicras-Mainar A, Morillo-Verdugo R. Potential interactions between pangenotypic direct-acting antivirals and concomitant cardiovascular therapies in patients with chronic hepatitis C virus infection. J Int Med Res. 2020;48(10):300060520964659
Keast SL, Holderread B, Cothran T, Skrepnek GH. Hepatitis C direct-acting antiviral treatment selection, treatment failure, and use of drug-drug interactions in a state medicaid program. J Manag Care Spec Pharm. 2019;25(11):1261–1267
Funding
This work was supported by the Investigator Sponsored Research of Gilead Sciences (Reference: IN-HK-987-6069).
Author information
Authors and Affiliations
Contributions
All authors were responsible for the study concept and design. VW-KH, TC-FY, Y-KT, and GL-HW were responsible for the acquisition and analysis of data, had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors were responsible for the interpretation of data, the drafting, and critical revision of the manuscript for important intellectual content.
Corresponding author
Ethics declarations
Conflict of interest
Terry Yip has served as an advisory committee member and a speaker for Gilead Sciences. Henry Chan is an advisor for AbbVie, Aligos, Aptorum, Arbutus, Hepion, Janssen, Gilead, GSK, Merck, Roche, Vaccitech, VenatoRx, Vir Biotechnology; and a speaker for Mylan, Gilead and Roche. Vincent Wong has served as an advisory committee member for 3 V-BIO, AbbVie, Allergan, Boehringer Ingelheim, Echosens, Gilead Sciences, Intercept, Janssen, Novartis, Novo Nordisk, Perspectum Diagnostics, Pfizer, TARGET-NASH and Terns; and a speaker for Bristol-Myers Squibb, Echosens, Gilead Sciences and Merck. He has also received a research grant from Gilead Sciences. Grace Wong has served as an advisory committee member for Gilead Sciences and Janssen, as a speaker for Abbott, Abbvie, Bristol-Myers Squibb, Echosens, Furui, Gilead Sciences, Janssen and Roche, and received research grant from Gilead Sciences. The other authors declare that they have no competing interests.
Ethical approval
The study protocol was approved by the Joint Chinese University of Hong Kong—New Territories East Cluster Clinical Research Ethics Committee.
Animal research (ethics)
Not applicable.
Consent to participate (ethics)
Because of the retrospective nature of this study and we will have no access to patient identifiers, written informed consent will be waived, subject to approval by the Joint CUHK-NTEC CREC.
Consent to publish (ethics)
All the authors are consent to publish the data.
Clinical trials registration
Not available.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hui, V.WK., Au, C.L., Lam, A.S.M. et al. Drug–drug interactions between direct-acting antivirals and co-medications: a territory-wide cohort study. Hepatol Int 16, 1318–1329 (2022). https://doi.org/10.1007/s12072-022-10402-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-022-10402-y