Abstract
Background
Severe alcoholic hepatitis (SAH) presenting as acute-on-chronic liver failure (ACLF) carries a high short-term mortality. Alteration of gut microbiota is a crucial component implicated in its pathogenesis, whose modulation has been suggested as a potential therapeutic tool. We evaluated the safety of fecal microbiota transplantation (FMT) and its efficacy in improving short-term survival and clinical severity scores in patients with SAH–ACLF.
Methods
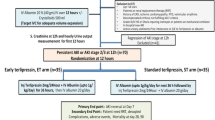
Thirty-three patients [13 in the FMT arm; 20 in the standard of care arm (SOC)] with SAH–ACLF were included in this open-label study. A single FMT session was administered as a freshly prepared stool suspension from pre-identified healthy family member stool donors through a nasojejunal tube. Patients were followed up on days 7, 28, and 90.
Results
Survival at 28 and 90 days was significantly better in the FMT arm (100% versus 60%, p = 0.01; 53.84% versus 25%, p = 0.02). Hepatic encephalopathy resolved in 100% versus 57.14% (FMT versus SOC, p = 0.11) patients, while ascites resolved in 100% versus 40% survivors (p = 0.04). Major adverse event rates, including spontaneous bacterial peritonitis and gastrointestinal bleeding, were similar in both groups (p = 0.77; p = 0.70). Median IL1beta decreased by 21.39% (IQR − 73.67 to 7.63) in the FMT group, whereas it increased in the SOC by 27.44% (IQR − 0.88 to 128.11) (p = 0.01). Percentage changes in bilirubin and ALT between baseline and day 7 emerged as predictors of 90-day mortality.
Conclusion
FMT is safe, improves short-term and medium-term survival, and leads to improvement in clinical severity scores in patients with SAH–ACLF.
Clinical trial number
NCT03827772 available from http://clinicaltrials.gov/ct2/show/NCT03827772
CTRI Reference number: CTRI/2019/02/017538 dated 7 February 2019.
Graphical abstract




Similar content being viewed by others
Data and code availability
Available.
Abbreviations
- ACLF:
-
Acute on chronic liver failure
- APASL:
-
Asian Pacific Association for the study of the liver
- ALT:
-
Alanine aminotransferase
- Anti-HBc:
-
Antibodies to hepatitis B core antigen
- AST:
-
Aspartate aminotransferase
- CANONIC:
-
Chronic Liver Failure (CLIF) Consortium Acute-on-Chronic Liver Failure in Cirrhosis
- CDI:
-
Clostridium difficile Infection
- CDR:
-
Cirrhosis dysbiosis ratio
- CLIF-C:
-
ACLF Chronic Liver Failure Consortium Acute-on-Chronic Liver Failure Score
- CLIF-C:
-
OF Chronic Liver Failure Consortium-Organ Failure score
- CLIF-SOFA:
-
Chronic Liver Failure-Sequential Organ Failure Assessment score
- CTP:
-
Child–Turcotte–Pugh score
- FMT:
-
Fecal microbiota transplantation
- GM:
-
Gut microbiota
- HAV:
-
Hepatitis A virus
- HBsAg:
-
Surface antigen for hepatitis B
- HCV:
-
Hepatitis C virus
- HE:
-
Hepatic encephalopathy
- HIV:
-
Human immunodeficiency virus
- IL:
-
Interleukin
- INR:
-
International normalized ratio
- LFT:
-
Liver function tests
- mDF:
-
Maddrey’s discriminant function
- MELD:
-
Score model for end-stage liver disease
- MELD-Na+ :
-
Score model for end-stage liver disease sodium score
- PAMPs:
-
Pathogen-associated molecular patterns
- PGIMER:
-
Postgraduate Institute of Medical Education and Research
- PRR:
-
Pattern recognition receptor
- SAH:
-
Severe alcoholic hepatitis
- SOC:
-
Standard of care
- VDRL:
-
Venereal Disease Research Laboratory test
References
Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology 2011;141:1572–1585
Gholam PM. Prognosis and prognostic scoring models for alcoholic liver disease and acute alcoholic hepatitis. Clin Liver Dis 2016;20:491–497
Thursz MR, Richardson P, Allison M, Austin A, Bowers M, Day CP, et al. Prednisolone or pentoxifylline for alcoholic hepatitis. N Engl J Med 2015;372:1619–1628
Mathurin P, Moreno C, Samuel D, Dumortier J, Salleron J, Durand F, et al. Early liver transplantation for severe alcoholic hepatitis. N Engl J Med 2011;365:1790–1800
Qin N, Yang F, Li A, Prifti E, Chen Y, Shao L, et al. Alterations of the human gut microbiome in liver cirrhosis. Nature 2014;513:59–64
Bull-Otterson L, Feng W, Kirpich I, Wang Y, Qin X, Liu Y, et al. Metagenomic analyses of alcohol induced pathogenic alterations in the intestinal microbiome and the effect of Lactobacillus rhamnosus GG treatment. PLoS ONE 2013;8(1):e53028
Bajaj JS, Heuman DM, Hylemon PB, Sanyal AJ, White MB, Monteith P, et al. The cirrhosis dysbiosis ratio defines changes in the gut microbiome associated with cirrhosis and its complications. J Hepatol 2014;60(5):940–947
Llopis M, Cassard AM, Wrzosek L, Boschat L, Bruneau A, Ferrere G, et al. Intestinal microbiota contributes to individual susceptibility to alcoholic liver disease. Gut 2016;65:830839
Purohit V, Bode JC, Bode C, Brenner DA, Choudhry MA, Hamilton F, et al. Alcohol, intestinal bacterial growth, intestinal permeability to endotoxin, and medical consequences: summary of a symposium. Alcohol 2008;42(5):349–361
Szabo G. Gut-liver axis in alcoholic liver disease. Gastroenterology 2015;148(1):30–36
Anand G, Zarrinpar A, Loomba R. Targeting dysbiosis for the treatment of liver disease. Semin Liver Dis 2016;36:37–47
Philips CA, Pande A, Shasthry SM, Jamwal KD, Khillan V, Chandel SS, et al. Healthy donor fecal microbiota transplantation in steroid ineligible severe alcoholic hepatitis—a pilot study. Clin Gastroenterol Hepatol 2017;15:600–602
Philips CA, Phadke N, Ganesan P, Augustine P. Healthy donor fecal transplant for corticosteroid non-responsive severe alcoholic hepatitis. BMJ Case Rep 2017
Philips CA, Phadke N, Ganesan K, Ranade S, Augustine P. Corticosteroids, nutrition, pentoxiphylline or fecal microbiota transplantation for severe alcoholic hepatitis. Indian J Gastroenterol 2018;37(3):215–225
Sarin SK, Pande A, Schnabl B. Microbiome as a therapeutic target in alcohol-related liver disease. J Hepatol 2019;70:260–272
Crabb DW, Bataller R, Chalasani NP, Kamath PS, Lucey M, Mathurin P, et al. Standard definitions and common data elements for clinical trials in patients with alcoholic hepatitis: recommendation from the NIAAA Alcoholic Hepatitis Consortia. Gastroenterology 2016;150:785–790
Sarin SK, Kumar A, Almeida JA, Chawla YK, Fan ST, Garg H, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the study of the liver (APASL). Hepatol Int 2009;3(1):269–282
Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology 2013;144(7):1426–1437.e14379
European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol 2018;S0168–8278(18):31966–31974
Sersté T, Cornillie A, Njimi H, Pavesi M, Arroyo V, Putignano A, et al. The prognostic value of acute-on-chronic liver failure during the course of severe alcoholic hepatitis. J Hepatol 2018;69(2):318–324
Gustot T, Jalan R. Acute-on-chronic liver failure in patients with alcohol-related liver disease. J Hepatol 2019;70:319–327
Forrest EH, Atkinson SR, Richardson P, Masson S, Ryder S, Thursz MR, et al. Prevalent acute-on-chronic liver failure and response to corticosteroids in alcoholic hepatitis. J Hepatol 2018;69:1200–1201
Gupta A, Dhiman RK, Kumari S, Rana S, Agarwal R, Duseja A, et al. Role of small intestinal bacterial overgrowth and delayed gastrointestinal transit time in cirrhotic patients with minimal hepatic encephalopathy. J Hepatol 2010;53:849–855
Quigley EM, Stanton C, Murphy EF. The gut microbiota and the liver. Pathophysiol Clin Impl J Hepatol 2013;58:1020–1027
Bajaj JS, Kassam Z, Fagan A, Gavis EA, Liu E, Cox IJ, et al. Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: a randomized clinical trial. Hepatology 2017;66:1727–1738
Hill DB, Marsano L, Cohen D, Allen J, Shedlofsky S, McClain CJ. Increased plasma interleukin-6 concentrations in alcoholic hepatitis. J Lab Clin Med 1992;119(5):547–552
McClain CJ, Cohen D, Dinarello CA, Cannon JG, Shedlofsky S, Kaplan AM. Seruminterleukin-1 (IL-1) activity in alcoholic hepatitis. Life Sci 1986;39:1479–1485
Dhiman RK, Rana B, Agrawal S, Garg A, Chopra M, Thumburu KK, et al. Probiotic VSL#3 reduces liver disease severity and hospitalization in patients with cirrhosis: a randomized, controlled trial. Gastroenterology 2014;147:1327–1337.e3
Louvet A, Naveau S, Abdelnour M, Ramond MJ, Diaz E, Fartoux L, et al. The Lille model: a new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology 2007;45:1348–1354
Jalan R, Saliba F, Pavesi M, et al. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J Hepatol 2014;61:1038–1047
Li H, Chen LY, Zhang N, et al. Characteristics, diagnosis and prognosis of acute-on-chronic liver failure in cirrhosis associated to hepatitis B. Sci Rep 2016;6:25487
DeFilipp Z, Bloom P, Soto M, Mansour M. Drug-resistant E. coli bacteremia transmitted by fecal microbiota transplant. N Engl J Med 2019;381(21):2043–2050
Gopalakrishnan V, Dozier EA, Glover MS, Novick S, Ford M, Morehouse C, et al. Engraftment of bacteria after fecal microbiota transplantation is dependent on both frequency of dosing and duration of preparative antibiotic regimen. Microorganisms 2021;9(7):1399
Trebicka J, Macnaughtan J, Schnabl B, Shawcross DL, Bajaj JS. The microbiota in cirrhosis and its role in hepatic decompensation. J Hepatol 2021;75:S67–S81
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
AS and AR: data compilation and manuscript writing; RKD: concept, manuscript writing, and editing; MPK: manuscript editing and revision; NV: statistical analysis; AD: manuscript editing and revision; SG: data compilation; MC: data extraction; ST: manuscript revision.
Corresponding author
Ethics declarations
Conflict of interest
Anima Sharma, Akash Roy, Madhumita Premkumar, Nipun Verma, Ajay Duseja, Sunil Taneja, Sandeep Grover, Madhu Chopra and Radha K. Dhiman declare that they have no conflict of interest.
Statement of human rights
The study has been approved by the institutional ethics committee and follows the ethical standards as laid down in the 1964 Declaration of Helsinki and revised in 2008.
Informed consent
Obtained.
Consent for publication
Yes.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sharma, A., Roy, A., Premkumar, M. et al. Fecal microbiota transplantation in alcohol-associated acute-on-chronic liver failure: an open-label clinical trial. Hepatol Int 16, 433–446 (2022). https://doi.org/10.1007/s12072-022-10312-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-022-10312-z