Abstract
Background and aims
Antiviral treatment with necleos(t)ide analogues contributes to histological improvement and virologic response in chronic hepatitis B (CHB) patients. However, whether adding pegylated interferon alpha2a (Peg-IFN-α-2a) can help additional clinical benefit, particularly on fibrosis regression was still unknown.
Methods
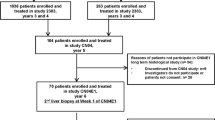
Chronic hepatitis B patients with pre-treatment biopsy-proven Ishak fibrosis score 2, 3 or 4 were randomly assigned to entecavir (ETV) alone or ETV plus Peg-IFN-α-2a (Peg-IFN-α-2a add-on) group (1:2 ratio). Post-treatment liver biopsy was performed at week 78. Fibrosis regression was defined as decrease in Ishak fibrosis score by ≥ 1 stage or predominantly regressive categorized by P–I–R score. Serum HBV DNA levels were assessed at baseline and every 26 weeks, while HBsAg and HBeAg were evaluated at baseline and every 52 weeks.
Results
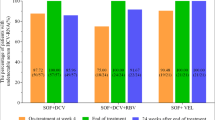
A total of 218 treatment-naive CHB patients were randomly assigned to ETV alone or Peg-IFN-α-2a add-on group. Totals of 155 patients (ETV alone: Peg-IFN-α-2a add-on, 47:108) were included in statistical analysis. Fibrosis regression rates were 68% (32/47) in the ETV alone and 56% (60/108) in Peg-IFN-α-2a add-on group (p = 0.144). Both groups showed a similar trend of virological suppression during the process of 104-week antiviral therapy (p = 0.132). HBeAg or HBsAg loss or seroconversion rates in the ETV alone group were lower than Peg-IFN-α-2a add-on group though without statistical significance.
Conclusions
Peg-IFN-α-2a add-on therapy did not yield additional fibrosis regression and virologic response than ETV alone therapy.



Similar content being viewed by others
Abbreviations
- CHB:
-
Chronic hepatitis B
- Peg-IFN-α-2a:
-
Pegylated interferon alpha2a
- ETV:
-
Entecavir
- P-I-R:
-
Predominantly progressive, indeterminate and predominately regressive
- HBV:
-
Hepatitis B virus
- HBsAg:
-
Hepatitis B surface antigen
- HBeAg:
-
Hepatitis B e antigen
- NAs:
-
Necleos(t)ide analogues
- IFNa:
-
Interferon-alpha
- AFP:
-
Alpha fetoprotein
- ULN:
-
Upper limit of normal
- AE:
-
Adverse event
- SAE:
-
Serious adverse event
- H & E:
-
Hematoxylin and eosin
- HAI:
-
Histology activity index
- LSM:
-
Liver stiffness measurement
- APRI:
-
Aminotransferase-to-platelet ratio index
- FIB-4:
-
Fibrosis-4 score
- IQR:
-
Interquartile range
- SD:
-
Standard deviation
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- PLT:
-
Platelet
- BMI:
-
Body mass index
- WBC:
-
White blood cell count
- ALB:
-
Albumin
- TB:
-
Total bilirubin
- INR:
-
International normalized ratio
References
World Health Organization. Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection: 2015 March. Geneva: World Health Organization; 2015.
Arvaniti V, D’Amico G, Fede G, et al. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology. 2010;139:1246–1256.e5.
Liaw YF, Sung JJ, Chow WC, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521–31.
Sun Y, Wu X, Zhou J, et al. Persistent low level of hepatitis b virus promotes fibrosis progression during therapy. Clin Gastroenterol Hepatol. 2020;18:2582–2591.e6.
Yuen MF, Wong DK, Fung J, et al. HBsAg Seroclearance in chronic hepatitis B in Asian patients: replicative level and risk of hepatocellular carcinoma. Gastroenterology. 2008;135:1192–9.
Sarin SK, Kumar M, Lau GK, et al. Asian–Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1–98.
Liang TJ, Block TM, McMahon BJ, et al. Present and future therapies of hepatitis B: from discovery to cure. Hepatology. 2015;62:1893–908.
Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560–99.
European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver. EASL. Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370–98.
Marcellin P, Gane E, Buti M, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381:468–75.
Papatheodoridis GV, Petraki K, Cholongitas E, et al. Impact of interferon-alpha therapy on liver fibrosis progression in patients with HBeAg-negative chronic hepatitis B. J Viral Hepat. 2005;12:199–206.
Chang TT, Gish RG, de Man R, et al. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med. 2006;354:1001–10.
Chang TT, Liaw YF, Wu SS, et al. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology. 2010;52:886–93.
Lau GKK, Piratvisuth T, Luo KX, et al. Peginterferon alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med. 2005;352:2682–95.
Janssen HL, van Zonneveld M, Senturk H, et al. Pegylated interferon alfa-2b alone or in combination with lamivudine for HBeAg-positive chronic hepatitis B: a randomised trial. Lancet. 2005;365:123–9.
Ning Q, Wu D, Wang GQ, Ren H, et al. Roadmap to functional cure of chronic hepatitis B: an expert consensus. J Viral Hepat. 2019;26:1146–55.
Brouwer WP, Xie Q, Sonneveld MJ, et al. Adding pegylated interferon to entecavir for hepatitis B e antigen-positive chronic hepatitis B: a multicenter randomized trial (ARES study). Hepatology. 2015;61:1512–22.
Hu P, Shang J, Zhang W, et al. HBsAg loss with peg-interferon alfa-2a in hepatitis B patients with partial response to nucleos(t) ide analog: new switch study. J Clin Transl Hepatol. 2018;6:25–34.
Lefkowitch JH. Scheuer’s liver biopsy interpretation. 9th ed. Edinburgh: Elsevier, 2015:11–6.
Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–9.
Sun Y, Zhou J, Wang L, et al. New classification of liver biopsy assessment for fibrosis in chronic hepatitis B patients before and after treatment. Hepatology. 2017;65:1438–50.
Theise ND, Jia J, Sun Y, et al. Progression and regression of fibrosis in viral hepatitis in the treatment era: the Beijing classification. Mod Pathol. 2018;31:1191–200.
Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–26.
Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32–6.
Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol. 2008;48:835–47.
Knodell RG, Ishak KG, Black WC, et al. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981;1:431–5.
Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289–93.
Buster EH, Hansen BE, Lau GK, et al. Factors that predict response of patients with hepatitis B e antigen-positive chronic hepatitis B to peginterferon-alfa. Gastroenterology. 2009;137:2002–9.
Sunbul M. Hepatitis B virus genotypes: global distribution and clinical importance. World J Gastroenterol. 2014;20:5427–34.
Cooksley WG, Piratvisuth T, Lee SD, et al. Peginterferon alpha-2a (40 kDa): an advance in the treatment of hepatitis B e antigen-positive chronic hepatitis B. J Viral Hepat. 2003;10:298–305.
European Association for the study of the liver. EASL clinical practice guidelines: management of chronic hepatitis B. J Hepatol. 2009;50:227–42.
Wiegand J, Hasenclever D, Tillmann HL. Should treatment of hepatitis B depend on hepatitis B virus genotypes? A hypothesis generated from an explorative analysis of published evidence. Antivir Ther. 2008;13:211–20.
Sun J, Xie Q, Tan D, et al. The 104-week efficacy and safety of telbivudine-based optimization strategy in chronic hepatitis B patients: a randomized, controlled study. Hepatology. 2014;59:1283–92.
Xie Q, Zhou H, Bai X, et al. A randomized, open-label clinical study of combined pegylated interferon Alfa-2a (40KD) and entecavir treatment for hepatitis B “e” antigen-positive chronic hepatitis B. Clin Infect Dis. 2014;59:1714–23.
Funding
This study was funded by the National Science and Technology Major Project (2018ZX10302204, 2018ZX10302204-004) and the National Natural Science Foundation of China (81800535 and 81670539).
Author information
Authors and Affiliations
Contributions
Study design: HY and JDJ. Liver biopsy assessment: TLW, HL, YMS, XYZ. Data collection: JLZ, YMS, XNW, TTM, XJO, BQW, SYC. Statistical analysis: SYC, YYK, SSW. Manuscript writing: SYC, JLZ. Critical revision of the manuscript: HY, YMS.
Corresponding authors
Ethics declarations
Conflicts of interest
All authors have nothing to disclose.
Ethical approval
The study was conducted in accordance with the principles enshrined in the Declaration of Helsinki and the Good Clinical Practices. The Ethics Committee of all participating centers approved the study protocol (2016-P2-021-02).
Informed consent
All patients gave written informed consent prior to their enrollment.
Consent for publication
All authors had access to the study data and reviewed and approved the final manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, S., Zhou, J., Wu, X. et al. Comparison of fibrosis regression of entecavir alone or combined with pegylated interferon alpha2a in patients with chronic hepatitis B. Hepatol Int 15, 611–620 (2021). https://doi.org/10.1007/s12072-021-10162-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-021-10162-1