Abstract
Background and aims
Rifaximin has been recommended as a prophylactic drug for hepatic encephalopathy (HE) and spontaneous bacterial peritonitis (SBP). This study aims to explore whether low-dose rifaximin can prevent overall complications and prolong survival in cirrhotic patients.
Methods
In this multi-centre randomized open-labelled prospective study, 200 patients with decompensated cirrhosis were randomly assigned at a ratio of 1:1. Patients in rifaximin group were administered 400 mg rifaximin twice daily for 6 months, and all other therapeutic strategies were kept unchanged in both groups as long as possible. The primary efficacy endpoints were the incidence of overall complications and liver transplantation-free survival. The secondary endspoints were the incidence of each major cirrhosis-related complication, as well as the Child–Pugh score and class.
Results
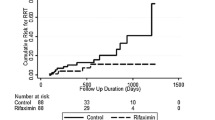
The major baseline characteristics were similar in the two groups except for HE. The cumulative incidence and frequency of overall complications were significantly lower in rifaximin group than in the control group (p < 0.001). Though liver transplantation-free survival was not significantly different between the two groups, subgroup analysis showed rifaximin markedly prolonged liver transplantation-free survival in patients with Child–Pugh score ≥ 9 (p = 0.007). Moreover, rifaximin markedly reduced the episodes of ascites exacerbation (p < 0.001), HE (p < 0.001) and gastric variceal bleeding (EGVB, p = 0.031). The incidence of adverse events was similar in the two groups.
Conclusion
Low-dose rifaximin significantly decreases the occurrence of overall complications, leading to prolonged survival in patients with advanced stages of cirrhosis in this trail. Further study should be carried out to compare the effect of this low-dose rifaximin with normal dose (1200 mg/day) rifaximin in preventing cirrhosis-related complications.
Clinical trial number
NCT02190357



Similar content being viewed by others
Abbreviations
- SBP:
-
Spontaneous bacterial peritonitis
- EGVB:
-
Oesophageal and gastric variceal bleeding
- HE:
-
Hepatic encephalopathy
- HRS:
-
Hepatorenal syndrome
- HCC:
-
Hepatocellular carcinoma
- GI:
-
Gastrointestinal
- AKI:
-
Acute kidney injury
- HBV:
-
Hepatitis B virus
- AIH:
-
Autoimmune hepatitis
- PBC:
-
Primary biliary cholangitis
- MELD:
-
Model For End-Stage Liver Disease
- PT:
-
Prothrombin time
- INR:
-
International normalized ratio
- ITT:
-
Intention-to-treat
- PHC:
-
Primary hepatic cancer
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- γ-GT:
-
γ-Glutamyl transferase
- ALP:
-
Alkaline phosphatase
- HVPG:
-
Hepatic venous pressure gradient
References
Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383:1749–61.
Wiest R, Albillos A, Trauner M, et al. Targeting the gut-liver axis in liver disease. J Hepatol. 2017;67:1084–103.
Tilg H, Cani PD, Mayer EA. Gut microbiome and liver diseases. Gut. 2016;65:2035–44.
Ponziani FR, Gerardi V, Pecere S, et al. Effect of rifaximin on gut microbiota composition in advanced liver disease and its complications. World J Gastroenterol. 2015;21:12322–33.
Peleman C, Camilleri M. Rifaximin, microbiota biology, and hepatic encephalopathy. Clin Transl Gastroenterol. 2016;7:e195.
Kang DJ, Kakiyama G, Betrapally NS, et al. Rifaximin exerts beneficial effects independent of its ability to alter microbiota composition. Clin Transl Gastroenterol. 2016;7:e187.
Bass NM, Mullen KD, Sanyal A, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362:1071–81.
Kimer N, Krag A, Møller S, et al. Systematic review with meta-analysis: the effects of rifaximin in hepatic encephalopathy. Aliment Pharmacol Ther. 2014;40:123–32.
Kamal F, Khan MA, Khan Z, et al. Rifaximin for the prevention of spontaneous bacterial peritonitis and hepatorenal syndrome in cirrhosis: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2017;29:1109–17.
Kang SH, Lee YB, Lee JH, et al. Rifaximin treatment is associated with reduced risk of cirrhotic complications and prolonged overall survival in patients experiencing hepatic encephalopathy. Aliment Pharmacol Ther. 2017;46:845–55.
Sharma BC, Sharma P, Lunia MK, et al. A randomized, double-blind, controlled trial comparing rifaximin plus lactulose with lactulose alone in treatment of overt hepatic encephalopathy. Am J Gastroenterol. 2013;108:1458–63.
Dong T, Aronsohn A, Gautham Reddy K, et al. Rifaximin decreases the incidence and severity of acute kidney injury and hepatorenal syndrome in cirrhosis. Dig Dis Sci. 2016;61:3621–6.
Ibrahim ES, Alsebaey A, Zaghla H, et al. Long-term rifaximin therapy as a primary prevention of hepatorenal syndrome. Eur J Gastroenterol Hepatol. 2017;29:1247–50.
Khokhar N, Qureshi MO, Ahmad S, et al. Comparison of once a day rifaximin to twice a day dosage in the prevention of recurrence of hepatic encephalopathy in patients with chronic liver disease. J Gastroenterol Hepatol. 2015;30:1420–2.
Zeng X, Tang XJ, Sheng X, et al. Does low-dose rifaximin ameliorate endotoxemia in patients with liver cirrhosis: a prospective study. J Dig Dis. 2015;16:665–74.
Vilstrup H, Amodio P, Bajaj J, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60:715–35.
Garcia-Tsao G, Abraldes JG, Berzigotti A, et al. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65:310–35.
Angeli P, Gines P, Wong F, et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. Gut. 2015;64:531–7.
Fukui H, Saito H, Ueno Y, et al. Evidence-based clinical practice guidelines for liver cirrhosis 2015. J Gastroenterol. 2016;51:629–50.
Vlachogiannakos J, Viazis N, Vasianopoulou P, et al. Long-term administration of rifaximin improves the prognosis of patients with decompensated alcoholic cirrhosis. J Gastroenterol Hepatol. 2013;28:450–5.
Flamm SL, Mullen KD, Heimanson Z, et al. Rifaximin has the potential to prevent complications of cirrhosis. Therap Adv Gastroenterol. 2018;11:1756284818800307.
Bajaj JS, Barrett AC, Bortey E, et al. Prolonged remission from hepatic encephalopathy with rifaximin: results of a placebo crossover analysis. Aliment Pharmacol Ther. 2015;41:39–45.
Goyal O, Sidhu SS, Kishore H. Minimal hepatic encephalopathy in cirrhosis- how long to treat? Ann Hepatol. 2017;16:115–22.
Sidhu SS, Goyal O, Parker RA, et al. Rifaximin vs. lactulose in treatment of minimal hepatic encephalopathy. Liver Int. 2016;36:378–85.
Lim YL, Kim MY, Jang YO, et al. Rifaximin and propranolol combination therapy is more effective than propranolol monotherapy for the reduction of portal pressure: an open randomized controlled pilot study. Gut Liver. 2017;11:702–10.
Zhang HL, Yu LX, Yang W, et al. Profound impact of gut homeostasis on chemically-induced pro-tumorigenic inflammation and hepatocarcinogenesis in rats. J Hepatol. 2012;57:803–12.
Hynicka LM, Silva KN. Probable rifaximin-induced neutropenia. Am J Health Syst Pharm. 2012;69:583–6.
Patel AS, Supan EM, Ali SN. Toxic epidermal necrolysis associated with rifaximin. Am J Health Syst Pharm. 2013;70:874–6.
Acknowledgements
The authors would like to thank the patients and their families for their contribution to this study.
Funding
The study was supported by an Emerging advanced technology joint research project from Shanghai Hospital Development Center (NO SHDC12016103), a Top-Level Clinical Discipline Project from Shanghai Pudong Health Committee (NO PWYgf2018-04), a Key Projects from Shanghai Science and Technology Committee (NO 17411950800) and two grants from the National Natural Science Foundation Committee of China (NO 81530019, 81770600).
Author information
Authors and Affiliations
Contributions
XZ and W-FX designed the research and drafted the manuscript. XZ, LY, XM, J-MX, X-ZS, C-QY, XZ and N-HL presided over the enrolment and exclusion of the patients. XS, H-GX, YL, J-WZ, C-ZH, JY, T-TL, W-JM and XX followed up the patients and collected the data. P-MS and Z-LY check the data. P-QW and Y-BG established the database and analysed the data statistically.
Corresponding author
Ethics declarations
Conflict of interest
Xin Zeng, Xia Sheng, Pei-Qin Wang, Hai-Guang Xin, Yi-Bin Guo,Yong Lin, Jia-Wei Zhong, Cheng-Zhi He, Jie Yin, Tao-Tao Liu, Wei-Juan Ma, Xiao Xiao, Pei-Mei Shi, Zong-Li Yuan, Ling Yang, Xiong Ma, Jian-Ming Xu, Xi-Zhong Shen, Chang-Qing Yang, Xuan Zhu, Nong-Hua Lv and Wei-Fen Xie declares that they have no conflict of interesting.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study protocol was reviewed and approved by the institutional review board or ethics committee at each centre.
Informed consent
Written informed consent was obtained from all patients.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zeng, X., Sheng, X., Wang, PQ. et al. Low-dose rifaximin prevents complications and improves survival in patients with decompensated liver cirrhosis. Hepatol Int 15, 155–165 (2021). https://doi.org/10.1007/s12072-020-10117-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-020-10117-y