Abstract
Purpose
A high rate of sustained viral response (SVR) in Koreans with chronic hepatitis C (CHC) is related to a favorable IL28B genotype. We compared two dosing strategies for peginterferon alfa-2a in Koreans with CHC and defined the combined effect of polymorphisms and dosing on the virological response.
Methods
A total of 178 treatment-naïve patients with CHC genotype 1 were prospectively enrolled. All patients were randomly assigned to treatment with one of two peginterferon alfa-2a regimens: 180 μg per week for 48 weeks (full-dose group) or 180 μg per week during the first 12 weeks followed by 135 μg per week for the next 36 weeks (dose-reduction group). Polymorphisms related to IL28B, ITPA, C20orf194 and SLC29A1 were studied.
Results
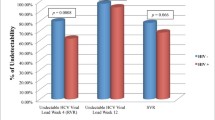
SVR rates did not differ between the full-dose and dose-reduction groups (56.5 and 51.2 %, respectively, p = 0.474). The frequency of additional reductions of the peginterferon dose because of adverse events was higher in the full-dose group than in the dose-reduction group. SVR rates in patients homozygous for the IL28B major allele were higher than those in patients for the other IL28B alleles. For patients with unfavorable IL28B genotypes, SVR was less likely to be achieved in the dose-reduction group than in the full-dose group.
Conclusions
In Koreans with HCV genotype 1, the virological response to treatment did not differ between a full dose and reduced dose (≥80 % of full dose) of peginterferon alfa-2a. However, in the patients with unfavorable IL28B genotypes, the full-dose treatment of peginterferon alfa-2a may be beneficial.


Similar content being viewed by others
Abbreviations
- HCV:
-
Hepatitis C virus
- SVR:
-
Sustained viral response
- SNP:
-
Single nucleotide polymorphism
- IL28B:
-
Interleukin 28B
- ITPA:
-
Inosine triphophatase
- CHC:
-
Chronic hepatitis C
- RVR:
-
Rapid virologic response
- EVR:
-
Early virologic response
- LD:
-
Linkage-disequlibrium
- ETVR:
-
End of treatment response
- ITT:
-
Intention to treatment
- PP:
-
Per protocol
References
Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology 2004;127:S35–S50
Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 2001;358:958–965
Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL Jr, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 2002;347:975–982
Shiffman ML, Ghany MG, Morgan TR, Wright EC, Everson GT, Lindsay KL, et al. Impact of reducing peginterferon alfa-2a and ribavirin dose during retreatment in patients with chronic hepatitis C. Gastroenterology 2007;132:103–112
Howell CD, Jeffers LS, Cassidy W, Reddy KR, Hu S, Lee JS. Peginterferon alfa-2a and ribavirin for chronic hepatitis C genotype 1 infections in black patients: safety, tolerability and impact on sustained virologic response. J Viral Hepat 2006;13:371–376
Iwasaki Y, Ikeda H, Araki Y, Osawa T, Kita K, Ando M, et al. Limitation of combination therapy of interferon and ribavirin for older patients with chronic hepatitis C. Hepatology 2006;43:54–63
Yu ML, Dai CY, Lin ZY, Lee LP, Hou NJ, Hsieh MY, et al. A randomized trial of 24- vs. 48-week courses of PEG interferon alpha-2b plus ribavirin for genotype-1b-infected chronic hepatitis C patients: a pilot study in Taiwan. Liver Int 2006;26:73–81
Dev AT, McCaw R, Sundararajan V, Bowden S, Sievert W. Southeast Asian patients with chronic hepatitis C: the impact of novel genotypes and race on treatment outcome. Hepatology 2002;36:1259–1265
Hepburn MJ, Hepburn LM, Cantu NS, Lapeer MG, Lawitz EJ. Differences in treatment outcome for hepatitis C among ethnic groups. Am J Med 2004;117:163–168
Kwon JH, Bae SH, Choi JY, Yoon SK, Byun KS, Paik SW, et al. Assessment of the efficacy of reducing peginterferon alpha-2a and ribavirin dose on virologic response in Koreans with chronic hepatitis C. Korean J Intern Med 2009;24:203–211
Lee H, Choi MS, Paik SW, Kim JH, Kim DY, Lee JH, et al. Peginterferon alfa-2a plus ribavirin for initial treatment of chronic hepatitis C in Korea. Korean J Hepatol 2006;12:31–40
McHutchison JG, Manns M, Patel K, Poynard T, Lindsay KL, Trepo C, et al. Adherence to combination therapy enhances sustained response in genotype-1-infected patients with chronic hepatitis C. Gastroenterology 2002;123:1061–1069
Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature 2009;461:399–401
Tanaka Y, Nishida N, Sugiyama M, Kurosaki M, Matsuura K, Sakamoto N, et al. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat Genet 2009;41:1105–1109
Thompson AJ, Fellay J, Patel K, Tillmann HL, Naggie S, Ge D, et al. Variants in the ITPA gene protect against ribavirin-induced hemolytic anemia and decrease the need for ribavirin dose reduction. Gastroenterology 2010;139:1181–1189
Nakagawa M, Naoya Sakamoto N, Watanabe T, Nishimura-Sakurai Y, Onozuka I, Azuma S, et al. Association of ITPA gene variation and serum ribavirin concentration with a decline in blood cell concentrations during pegylated interferon-alpha plus ribavirin therapy for chronic hepatitis C. Hepatol Int 2013;7:153–161
Tsubota A, Shimada N, Yoshizawa K, Furihata T, Agata R, Yumoto Y, et al. Contribution of ribavirin transporter gene polymorphism to treatment response in peginterferon plus ribavirin therapy for HCV genotype 1b patients. Liver Int 2012;32:826–836
Reddy KR, Hoofnagle JH, Tong MJ, Lee WM, Pockros P, Heathcote EJ, et al. Racial differences in responses to therapy with interferon in chronic hepatitis C. Consensus Interferon Study Group. Hepatology 1999;30:787–793
Missiha S, Heathcote J, Arenovich T, Khan K. Impact of Asian race on response to combination therapy with peginterferon alfa-2a and ribavirin in chronic hepatitis C. Am J Gastroenterol 2007;102:2181–2188
Muir AJ, Bornstein JD, Killenberg PG. Peginterferon alfa-2b and ribavirin for the treatment of chronic hepatitis C in blacks and non-Hispanic whites. N Engl J Med 2004;350:2265–2271
Rangnekar AS, Fontana RJ. Meta-analysis: IL-28B genotype and sustained viral clearance in HCV genotype 1 patients. Aliment Pharmacol Ther 2012;36:104–114
Sinn DH, Kim YJ, Lee ST, Gwak GY, Choi MS, Lee JH, et al. Association of a single nucleotide polymorphism near the interleukin-28B gene with response to hepatitis C therapy in Asian patients. J Gastroenterol Hepatol 2011;26:1374–1379
Lyoo K, Song MJ, Hur W, Choi JE, Hong SW, Kim CW, et al. Polymorphism near the IL28B gene in Korean hepatitis C virus-infected patients treated with peg-interferon plus ribavirin. J Clin Virol 2011;52:363–366
Huang CF, Huang JF, Yang JF, Hsieh MY, Lin ZY, Chen SC, et al. Interleukin-28B genetic variants in identification of hepatitis C virus genotype 1 patients responding to 24 weeks peginterferon/ribavirin. J Hepatol 2012;56:34–40
Lin CY, Chen JY, Lin TN, Jeng WJ, Huang CH, Huang CW, et al. IL28B SNP rs12979860 is a critical predictor for on-treatment and sustained virologic response in patients with hepatitis C virus genotype-1 infection. PLoS One 2011;6:e18322
Jung YK, Kim JH, Ahn SM, Yang JW, Park SJ, Kim JW, et al. Role of interleukin 28B-related gene polymorphisms in chronic hepatitis C and the response to antiviral therapy in Koreans. J Clin Gastroenterol 2013;47:644–650
Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology 2009;49:1335–1374
The national Center for Biotechnology Information. dbSNP. http//www.ncbi.nlm.nih.gov/projects/SNP/. Accessed 30 Nov 2012
Fellay J, Thompson AJ, Ge D, Gumbs CE, Urban TJ, Shianna KV, et al. ITPA gene variants protect against anaemia in patients treated for chronic hepatitis C. Nature 2010;464:405–408
Liu CH, Liang CC, Liu CJ, Tseng TC, Lin CL, Yang SS, et al. Interleukin 28B genetic polymorphisms and viral factors help identify HCV genotype-1 patients who benefit from 24-week pegylated interferon plus ribavirin therapy. Antivir Ther 2012;17:477–484
Acknowledgements
Kwon JH, Bae SH, Lee YJ planned the study design and analyzed and interpreted the data. JH Kwon drafted the manuscript. HY Yim supported the randomization of patients and statistical analysis. YJ Lee, JG Shin and JS Choi performed the SNP study. All the authors prospectively enrolled and treated patients and acquired the data. All authors had access to the study data and reviewed and approved the final manuscript. This study was supported by a grant from the research supporting program of the Korean Association for the Study of the Liver.
Conflict of interest
Jung Hyun Kwon, Si Hyun Bae, Youn Jae Lee, Jin-Woo Lee, Young Seok Kim, Jae Seok Hwang, Won Young Tak, Jeong Won Jang, Byung Seok Lee, June Sung Lee, Chun Kyon Lee, Soon Koo Baik, Neung Hwa Park, Tae Hee Lee, Dong Joon Kim, Jae-Seok Choi, Jae-Gook Shin and Hyeon Woo Yim declare that they have no conflict of interest.
Ethical standard
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and the Helsinki Declaration of 1975, as revised in 2008. Informed consent was obtained from all patients for inclusion in the study.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Kwon, J.H., Bae, S.H., Lee, Y.J. et al. The virological response in Koreans infected with HCV genotype 1 did not differ between groups treated with a full dose or reduced dose (≥80 % full dose) of peginterferon alfa-2a: a prospective randomized multicenter trial. Hepatol Int 7, 1000–1009 (2013). https://doi.org/10.1007/s12072-013-9472-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-013-9472-x