Abstract
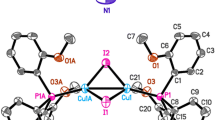
Formation of cationic and neutral CuI cluster MOFs have been reported starting from tridentate phosphoramide ligands, [\(\hbox {(NHR)}_{3}\hbox {P}=\hbox {E}\)] (\(\hbox {L}^{1}\): \(\hbox {R} = \hbox {3-aminoquinolinyl (AQ)}\), \(\hbox {E} = \hbox {S}\); \(\hbox {L}^{2}\): \(\hbox {R} = \hbox {3-pyridyl (PY)}\), \(\hbox {E} = \hbox {S}\); \(\hbox {L}^{3}\): \(\hbox {R} = \hbox {3-aminoquinolinyl (AQ)}\), \(\hbox {E} = \hbox {O}\)). By utilizing \(\hbox {L}^{1}\), a cationic 2D-MOF \(\{[(\hbox {L}^{1})_{2}\hbox {(Cu}_{6}\hbox {I}_{5})]\hbox {(OH)}\cdot \hbox {3DMF}\cdot \hbox {4MeOH}\}_{\mathrm{n}}\), 1 containing a rugby ball shaped discrete \(\hbox {Cu}_{6}\hbox {I}_{5}\) cluster has been reported earlier. Formation of a new 3D-MOF \(\{[(\hbox {L}^{2})_{2}(\hbox {Cu}_{6}\hbox {I}_{4})]\hbox {(OH)}_{2}\cdot \hbox {2DMF}\}_{\mathrm{n}}\) containing a Zintl type \([(\hbox {Cu}_{6}\hbox {I}_{4})^{2+}]_{\mathrm{n}}\) cluster chains is reported in this paper. A neutral cluster MOFs 3 with formula unit of \(\{[\hbox {Cu}_{4}\hbox {I}_{4}\hbox {L}^{3}(\hbox {CH}_{3}\hbox {CN})]\cdot \hbox {2DMF}\cdot 3\hbox {H}_{2}\hbox {O}\}_{\mathrm{n}}\) has been prepared from the ligand \(\hbox {L}^{3}\). Formation of the smaller \(\hbox {Cu}_{4}\hbox {I}_{4}\) clusters in the MOF 3 is due to the presence of a MeCN ligation at one of the Cu(I) atoms which not only precludes the extension of the assembly in three dimension but also reduces the size of the obtained cluster. Unlike 1 which showed a ligand-assisted thermochromism, photophysical studies on the 3D-MOF 2 exhibited green phosphorescence at both 298 K and 77 K. The occurrence of the phosphorescence at 77 K in 2 is due to triplet cluster centered (\({}^3\hbox {CC}\)) excited state of the cluster as there is no ligand-centered transition observed at 298 K. The 2D-MOF 3 does not show any characteristic luminescence behavior as the presence of the acetonitrile coordination at one of the Cu(I) ion is believed to quench the emission by non-radiative pathways. Further, luminescence quenching experiments on 1 and 2 with aromatic nitro-analytes showed a very high sensing selectivity for picric acid (TNP) over other aromatic nitro-analytes.
Graphical Abstract

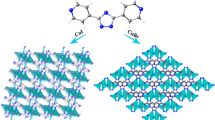
SYNOPSIS: Employing tridentate phosphoramide ligands containing 3-aminoquinolyl and 3-pyridyl moieties, new examples of cluster MOFs containing cationic and neutral copper(I) iodide clusters were prepared. The MOF 2 contains a rugby ball-shaped, edge-fused polymeric \(\{[\hbox {Cu}_{6}\hbox {I}_{4}]^{2+}\}_{\mathrm{n}}\) clusters, whereas the MOF 3 contains a cubane-type discrete \(\hbox {Cu}_{4}\hbox {I}_{4}\) neutral cluster. Photophysical studies on the cationic complexes showed interesting phosphorescence emission and selective detection of picric acid.










Similar content being viewed by others
References
(a) Shan X-C, Jiang F-L, Yuan D-Q, Zhang H-B, Wu M-Y, Chen L, Wei J, Zhang S-Q, Pan J and Hong M-C 2013 A multi-metal-cluster MOF with \(\text{Cu}_{4}\text{I}_{4}\) and \(\text{Cu}_{6}\text{S}_{6}\) as functional groups exhibiting dual emission with both thermochromic and near-IR character Chem. Sci. 4 1484; (b) Zhan S-Z, Li M, Zhou X-P, Wang J-H, Yang J-R and Li D 2011 When \(\text{Cu}_{4}\text{I}_{4}\) cubane meets \(\text{Cu}_{3}(\text{pyrazolate})_{3}\) triangle: Dynamic interplay between two classical luminophores functioning in a reversibly thermochromic coordination polymer Chem. Commun. 47 12441; (c) Amoore J J M, HantonL R and Spicer M D 2003 Banded ribbons of \(\text{Cu}_{6}\text{I}_{6}\) hexamers and multimodal thioetherpyrazine ligands linked by self-complementary \(\text{N}\cdots \text{H}{-}\text{C}\) synthons Dalton Trans. 1056; (d) Peng R, Wu T and Li D 2005 A chiral coordination polymer containing copper(I) iodide layer composed of intersecting CuI\(_\text{n}\) helices CrystEngComm 7 595; (f) Tzeng B-C and Chang T-Y 2009 Cryst. Growth Des. 9 5343; (e) Deshmukh M S, Yadav A, Pant R and Boomishankar R 2015 Thermochromic and Mechanochromic Luminescence Umpolung in Isostructural Metal–Organic Frameworks Based on \(\text{Cu}_{6}\text{I}_{6}\) Clusters Inorg. Chem. 54 1337
(a) Kitagawa H, Ozawa Y and Toriumi K 2010 Flexibility of cubane-like \(\text{Cu}_{4}\text{I}_{4}\) framework: Temperature dependence of molecular structure and luminescence thermochromism of \([\text{Cu}_{4}\text{I}_{4}(\text{PPh}_{3})_{4}]\) in two polymorphic crystalline states Chem. Commun. 46 6302; (b) Shan X-C, Jiang F-L, Yuan D-Q, Wu M-Y, Zhang S-Q and Hong M-C 2012 The unusual thermochromic NIR luminescence of Cu(I) clusters: Tuned by Cu–Cu interactions and packing modes Dalton Trans. 41 9411
(a) Kang Y, Wang F, Zhang J and Bu X 2012 Luminescent MTN-Type cluster–organic framework with 2.6 nm cages J. Am. Chem. Soc. 134 17881; (b) Prochowicz D, Justyniak I, Kornowicz A, Kaczorowski T, Kaszkur Z and Lewiński 2012 Construction of a Porous Homochiral Coordination Polymer with Two Types of \(\text{Cu}_\text{n}\text{I}_\text{n}\) Alternating Units Linked by Quinine: A Solvothermal and a Mechanochemical Approach Chem. Eur. J. 18 7367
(a) Kim,T H, Shin Y W, Jung J H, Kim J S and Kim J 2008 Crystal-to-Crystal Transformation between Three CuI Coordination Polymers and Structural Evidence for Luminescence Thermochromism Angew. Chem. Int. Ed. 47 685; (b) Liu Z, Djurovich P I, Whited M T and Thompson M E 2012 \(\text{Cu}_{4}\text{I}_{4}\) Clusters Supported by \(\text{P}^{\wedge}\text{N-type}\) Ligands: New Structures with Tunable Emission Colors Inorg. Chem. 51 230; (c) Blake A J, Brooks N R, Champness N R, Crew M, Davison A, Fenske D, Gregory D H, Hanton L R, Hubberstey P and Schröder M 2001 Topological isomerism in coordination polymers Chem. Commun. 1432; (d) Hu S and Tong M-L 2005 Rational design and construction of the first tetrahedral net with photoluminescent \(\text{Cu}_{4}\text{I}_{4}\) cubane cluster as the tetrahedral node Dalton Trans. 1165; (e) Chen Y, Li H-X, Liu D, Liu L-L, Li N-Y, Ye H-Y, Zhang Y and Lang J-P 2008 Solvent effects on the assembly of \([\text{Cu}_{2}\text{I}_{2}]\)-or \([\text{Cu}_{4}\text{I}_{4}]\)-based coordination polymers: Isolation, structures, and luminescent properties Cryst. Growth Des. 8 3810; (f) Pike R D, Reinecke B A, Dellinger M E, Wiles A B, Harper J D, Cole J R, Dendramis K A, Borne B D, Harris J L and Pennington W T 2004 Bicyclic phosphite esters from pentaerythritol and dipentaerythritol: New bridging ligands in organometallic and inorganic chemistry Organometallics 23 1986
(a) Lu J Y 2003 Crystal engineering of Cu-containing metal–organic coordination polymers under hydrothermal conditions Coord. Chem. Rev. 246 327; (b) Ouellette W, Prosvirin A V, Chieffo V, Dunbar K R, Hudson B and Zubieta J 2006 Solid-state coordination chemistry of the Cu/triazolate/X system (X= F-, Cl-, Br-, I-, OH-, and \(\text{SO}_{4}^{2-}\)) Inorg. Chem. 45 9346; (c) Wu T, Li M, Li D and Huang X-C 2008 Anionic \(\text{Cu}_{{\rm n}}\text{I}_{{\rm n}}\) cluster-based architectures induced by in situ generated N-alkylated cationic triazolium salts Cryst. Growth Des. 8 568; (d) Prajapati R K and Verma S 2011 Adenine coordination around a \(\text{Cu}_{6}\text{I}_{6}\) core Inorg. Chem. 50 3180
(a) Gu X and Xue D 2007 3D Coordination Framework \([\text{Ln}_{4}(\mu ^{3}\text{-OH})_{2}\text{Cu}_{6}\text{I}_{5}\text{(IN)}_{8}\text{(OAc)}_{3}]\) (IN = Isonicotinate): Employing 2D Layers of Lanthanide Wheel Clusters and 1D Chains of Copper Halide Clusters Inorg. Chem. 46 5349; (b) Wang F, Wu X-Y, Yu R-M and Lu C-Z 2012 An unprecedented \([\text{Cu}_{7}\text{I}_{4}]^{3+}\) cationic cluster-based metal–organic framework (MOF) including 1D nanochannels Inorg. Chem. Commun. 17 169; (c) Wang X-L, Qin C, Wang E-B, Su Z-M, Li Y-G and Xu L 2006 Self-Assembly of Nanometer-Scale \([\text{Cu}_{24}\text{I}_{10}\text{L}_{12}]^{14+}\) Cages and Ball-Shaped Keggin Clusters into a (4,12)-Connected 3D Framework with Photoluminescent and Electrochemical Properties Angew. Chem. Int. Ed. 45 7411; (d) Li M, Li Z and Li D 2008 Unprecedented cationic copper (I)–iodide aggregates trapped in “click” formation of anionic-tetrazolate-based coordination polymers Chem. Commun. 3390; (e) Cheng J-W, Zhang J, Zheng S-T and Yang G-Y 2008 Linking two distinct layered networks of nanosized \(\{{\rm Ln18}\}\) and \(\{{\rm Cu24}\}\) wheels through isonicotinate ligands Chem. Eur. J. 14 88; (f) Wang F, Wu X-Y, Yu R-M and Lu C-Z 2012 Inorg. Chem. Commun. 19 70; (g) Wang Y-L, Zhang N, Liu Q-Y, Shan Z-M, Cao R, Wang M-S, Luo J-J and Yang E-L 2011 Diversity of Architecture of Copper(I) Coordination Polymers Constructed of Copper(I) Halides and 4-Methyl-1,2,4-Triazole-3-Thiol (Hmptrz) Ligand: Syntheses, Structures, and Luminescent Properties Cryst. Growth Des. 11 130
(a) Gupta A K, Chipem F A S and Boomishankar R 2012 A 2-pyridyl (py) attached phosphine imine \(\text{[P(Npy)(NHpy)}_{3}]\) and an imido phosphinate ion \(\text{[P(Npy)}_{2}\text{(NHpy)}_{2}]^{-}\) in its Ag (I) complex Dalton Trans. 41 1848; (b) Gupta A K, Steiner A and Boomishankar R 2012 Tri-, hepta-and octa-nuclear Ag (I) complexes derived from 2-pyridyl-functionalized tris(amido)phosphate ligand Dalton Trans. 41 9753; (c) Gupta A K, Nicholls J, Debnath S, Rosbottom I, Steiner A and Boomishankar R 2011 Organoaminophosphoniumcations as building blocks for hierarchical supramolecular assemblies Cryst. Growth Des. 11 555
Yadav A, Srivastava A K, Balamurugan A and Boomishankar R 2014 A cationic copper (I) iodide cluster MOF exhibiting unusual ligand assisted thermochromism Dalton Trans. 43 8166
(a) Lan A, Li K, Wu H, Olson D H, Emge T J, Ki W, Hong M and Li J 2009 A luminescent microporous metal–organic framework for the fast and reversible detection of high explosives Angew. Chem. Int. Ed. 48 2334; (b) Nagarkar S S, Joarder B, Chaudhari A K, Mukherjee S and Ghosh S K 2013 Highly selective detection of nitro explosives by a luminescent metal–organic framework Angew. Chem. Int. Ed. 52 2881; (c) Zhang Z, Xiang S, Rao X, Zheng Q, Fronczek F R, Quan G and Chen B 2010 A rod packing microporous metal–organic framework with open metal sites for selective guest sorption and sensing of nitrobenzene Chem. Comm. 46 7205
(a) Germain M E and Knapp M J 2009 Optical explosives detection: From color changes to fluorescence turn-on Chem. Soc. Rev. 38 2543; (b) Liu T H, Ding L P, Zhao K R, Wang W L and Fang Y 2012 Single-layer assembly of pyrene end-capped terthiophene and its sensing performances to nitroaromatic explosives J. Mater. Chem. 22 1069; (c) Bhalla V, Gupta A and Kumar M 2012 Fluorescent nanoaggregates of pentacenequinone derivative for selective sensing of picric acid in aqueous media Org. Lett. 14 3112; (d) Roy B, Bar A K, Gole B and Mukherjee P S 2013 Fluorescent tris-imidazolium sensors for picric acid explosive J. Org. Chem. 78 1306; (e) Bhalla V, Gupta A, Kumar M, Rao D S S and Prasad S K 2013 Self-assembled pentacenequinone derivative for trace detection of picric acid ACS Appl. Mater. Interfaces 5 672; (f) Peng Y, Zhang A-J, Dong M and Wang Y-W 2011 A colorimetric and fluorescent chemosensor for the detection of an explosive 2, 4, 6-trinitrophenol (TNP) Chem. Commun. 47 4505; (g) Toal S J and Trogler W C 2006 Polymer sensors for nitroaromatic explosives detection J. Mater. Chem. 16 2871
(a) Yadav A and Boomishankar R 2015 Concentration dependentratiometric turn-on selective fluorescence detection of picric acid in aqueous and non-aqueous media RSC Adv. 5 3903; (b) Li N, Jiang F, Chen L, Li X, Chen Q and Hong M 2011 From discrete octahedral nanocages to 1D coordination polymer: Coordination-driven a single-crystal-to-single-crystal transformation via anion exchange Chem. Commun. 47 2327
Sheldrick G M 2015 Crystal structure refinement with SHELXL Acta Crystallogr. C 71 3
Frisch M J et al., 2004 Gaussian 03 Revision B.05 Gaussian Inc. Wallingford CT
Becke A D 1993 Density functional thermochemistry. III. The role of exact exchange J. Chem. Phys. 98 5648
Hay P J, Wadt W R 1985 Ab initio effective core potentials for molecular calculations: Potentials for K to Au including the outermost core orbitals J. Chem. Phys. 82 270
O’Keeffe M and Yaghi O M 2012 Deconstructing the crystal structures of metal–organic frameworks and related materials into their underlying nets Chem. Rev. 112 675
(a) Kyle K R, Ryu C K, DiBenedetto J A and Ford P C 1991 Photophysical studies in solution of the tetranuclear copper (I) clusters \(\text{Cu}_{4}\text{I}_{4}\text{L}_{4}\) (L= pyridine or substituted pyridine) J. Am. Chem. Soc. 113 2954; (b) Ford P C, Cariati E and Bourassa J 1999 Photoluminescence properties of multinuclear copper (I) compounds Chem. Rev. 99 3625; (c) Lindsay E and Ford P C 1996 Excited state absorption spectra of the tetranuclear cuprous iodide cluster \(\text{Cu}_{4}\text{I}_{4}\text{(py)}_{4}\) and related species Inorg. Chim. Acta 242 51; (d) Kyle K R and Ford P C 1989 Dynamic quenching of the metal-to-ligand charge-transfer excited state of \(\text{Cu}_{4}\text{I}_{4}\text{(pyridine)}_{4}\). Exciplex formation and self-quenching J. Am. Chem. Soc. 111 5005; (e) Rath N P, Holt E M and Tanimura K 1986 Fluorescent copper (I) complexes: Correlation of structural and emission characteristics of \([\{\text{Cul(quin)}_{2}\}_{2}]\) and \([\text{Cu}_{4}\text{I}_{4} \text{(quin)}_{4}]\)(quin= quinoline) J. Chem. Soc., Dalton Trans. 2303; (f) Hu G, Mains G J and Holt E M 1995 Correlation of structure and emission in solid state copper (I) complexes; \([\text{Cu}_{4}\text{I}_{4}(\text{CH}_{3}\text{CN})_{2}\text{(L)}_{2}]\), L= aniline derivative Inorg. Chim. Acta 240 559; (g) Rath N P, Holt E M and Tanimura K 1985 Fluorescent copper(I) complexes: Structural and spectroscopic characterization of bis(p-toluidine)bis(acetonitrile)tetraiodotetracopper and bis[(p-chloroaniline)(acetonitrile)diiododicopper] tetrameric complexes of mixed-ligand character Inorg. Chem. 24 3934
Safko J P, Kuperstock J E, McCullough S M, Noviello A M, Li X, Killarney J P, Murphy C, Patterson H H, Bayse C A and Pike R D 2012 Network formation and photoluminescence in copper (I) halide complexes with substituted piperazine ligands Dalton Trans. 41 11663
Ryu C K, Kyle K R and Ford P C 1991 Photoluminescence properties of the copper (I) chloride clusters \(\text{Cu}_{4}\text{Cl}_{4}\text{L}_{4}\) (L= pyridine, substituted pyridine, or saturated amine) Inorg. Chem. 30 3982; (b) De Ahna H D and Hardt H D 1972 Fluoreszenz ünd Fluoreszenz-Thermochromie bei Alkyl-pyridinokupfer (I)-Halogeniden Z. Anorg. Chem. 387 61; (c) Vitale M, Palke W E and Ford P C 1992 Origins of the double emission of the tetranuclear copper (I) cluster \(\text{Cu}_{4}\text{I}_{4}\text{(pyridine)}_{4}\): An ab initio study J. Phys. Chem. 96 8329; (d) Henary M and Zink J I 1989 Photoluminescence of cubic mixed-metal tetrameric clusters J. Am. Chem. Soc. 111 7404
(a) Ma Y, Li H, Shan P and Wang L 2012 Highly selective and sensitive fluorescent paper sensor for nitroaromatic explosive detection Anal. Chem. 84 8415; (b) Bhalla V, Kaur S, Vij V and Kumar M 2013 Mercury-modulated supramolecular assembly of a hexaphenylbenzene derivative for selective detection of picric acid Inorg. Chem. 52 4860; (c) Safko J P, Kuperstock J E, McCullough S M, Noviello A M, Li X, Killarney J P, Murphy C, Patterson H H, Bayse C A and Pike R D 2012 Network formation and photoluminescence in copper (I) halide complexes with substituted piperazine ligands Dalton Trans. 41 11663
Acknowledgements
This work was supported by SERB, India via Grant No. EMR/2016/000614 (R.B.). A.Y. thanks the CSIR, India and M.S.D. thanks the UGC, India for the fellowship. The High Energy Materials Research Laboratory, Pune is acknowledged for providing the samples of the Nitroaromatic compounds to IISER, Pune.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Prof. K C Kumara Swamy on the occasion of his $$60^{\mathrm{th}}$$ 60 th Birthday.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yadav, A., Deshmukh, M.S. & Boomishankar, R. Cationic and neutral copper(I) iodide cluster MOFs derived from tridentate N-donor functionalized P(V) ligands: synthesis, structure and photophysical properties. J Chem Sci 129, 1093–1103 (2017). https://doi.org/10.1007/s12039-017-1284-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-017-1284-4