Abstract
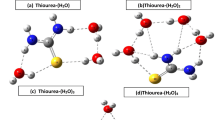
Ab initio and DFT methods have been employed to study the hydrogen bonding ability of formamide, urea, urea monoxide, thioformamide, thiourea and thiourea monoxide with one water molecule and the homodimers of the selected molecules. The stabilization energies associated with themonohydrated adducts and homodimers’ formation were evaluated at B3LYP/6-311++G** and MP2/6-311++G** levels. The energies were corrected for zero-point vibrational energies and basis set superposition error using counterpoise method. Atoms in molecules study has been carried out in order to characterize the hydrogen bonds through the changes in electron density and laplacian of electron density. A natural energy decomposition and natural bond orbital analysis was performed to understand the nature of hydrogen bonding.

Sixteen hydrogen bonded adducts of formamide, urea and urea monoxide with one water molecule and their homodimers have been optimized at B3LYP/6-311++G** and MP2/6-311++G** levels. Monohydrated Adducts and homodimers formation with the corresponding thio-analogs were also studied for comparative purpose. Atoms in molecules study has been carried out in order to characterize the hydrogen bonds. A natural energy decomposition and natural bond orbital analysis were performed to understand the nature of hydrogen bonding.


Similar content being viewed by others

References
Jeffrey G A 1997 In An Introduction to Hydrogen Bonding (USA: Oxford University Press)
Desiraju G R and Stenier T 1999 In The Weak Hydrogen Bond (Oxford: Oxford University Press)
Bandhopadhyay I, Lee H M and Kim K S 2005 J. Phys. Chem. A. 109 1720
Sobczyk L, Grabowski S and Krygowski T M 2005 Chem. Rev. 105 3513
Hinton J F and Harpool R D 1997 J. Am. Chem. Soc. 99 349
Jaeisen P G and Stevens W J 1986 J. Chem. Phys. 84 3271
Engdahl A and Nelander B 1993 J. Chem. Phys. 99 4894
Sim F and St-Amant A 1992 J. Am. Chem. Soc. 114 439
Wang X C, Facelli J C and Simons J 1993 Int. J. Quantum. Chem. 45 123
Liu T, Li H, Huang M, Duan Y and Wang Z 2008 J. Phys. Chem. A. 112 5436
Urban J J, Tillman B G and Cronin W A 2006 J. Phys. Chem. A. 110 11120
Pliego J R Jr 2004 Chem. Phy. 306 273
Cordeiro M A M, Santana W P, Cusinato R and Cordeiro J M M 2006 J. Mol. Struct. (THEOCHEM) 759 159
Sakai D, Mastuda Y, Hachiya M, Mori M, Fujii A and Mikami N 2008 J. Phys. Chem. A. 112 6840
Del Bene J E, Alkorta I and Elguero J 2008 J. Phys. Chem. A. 112 6338
Taha A N and True N S 2000 J. Phys. Chem. A. 104 2985
Angelina E L and Peruchena N M 2011 J. Phys. Chem. A. 115 4701
Espinosa E and Molins E 2000 J. Chem. Phys. 113 5686
Sunita S S, Rohini N K, Kulkarni M G, Nagaraju M and Sastry G N 2006 J. Am. Chem. Soc. 128 7752
Sunita S S, Rohini N K, Kulkarni M G, Nagaraju M and Sastry G N 2007 Macromolecules 40 1824
Nagaraju M and Sastry G N 2010 Int. J. Quantum. Chem. 110 1994
Nenitescu K D 1962 In Organicheskaya Khimiya (Organic Chemistry). Izdatestvo Innostrannoy Literatury, Academician Kabachnick, MI (ed.) (Moscow: Publishing of foreign literature) Vol. 1 p 815
Zhang R, Zhao G and Wu W 2009 Chin. J. Chem. Phys. 22 511
Lee K, Benson D R, and Kuczera K 2000 Biochemistry 39 13737
Gao J, Pavelites J J and Habibollazadeh D 1996 J. Phys. Chem. A. 100 2689
Vishnyakov A, Lyubartsev A P and Laaksonen A 2001 J. Phys. Chem. A. 105 1702
Finer E G, Franks F and Tait M J 1972 J. Am. Chem. Soc. 94 4424
Hoccart X and Turrel G J 1993 J. Chem. Phys. 99 8498
Keuleers R, Rousseau B, Alsenoy C V and Desseyn H O 1999 J. Phys. Chem. A. 103 462
Ramondo F, Bencienni L, Caminiti R, Pieretti A and Gontrani L 2007 Phys. Chem. Chem. Phys. 9 2206
Burton R C, Ferrari E S, Davey R J, Hopwood J, Qualey M J, Finney J L and Bowron D T 2008 Cryst. Growth Des. 8 1559
Siu D and Koga Y 2005 J. Phys. Chem. B. 109 16886
Lee M and van der Vegt N F A 2006 J. Am. Chem. Soc. 128 4948
Fong C, Wells D, Krodkiewska I, Hartley P G and Drummond C J 2006 Chem. Mater. 18 594
Koga Y, Miyazaki Y, Nagano Y and Inaba A 2008 J. Phys. Chem. B. 112 11341
Weiqn Z, Wen Y and Lihua Qiu 2005 J. Mol. Struct. (THEOCHEM) 730 133
Vazquez L, Salvarezza R C and Arvia A 1997 J. Phys. Rev. Lett. 79 709
Kim K, Lin Y T and Mosher H S 1988 Tetrahedron. Lett. 29 3183
Maryanoff C A, Stanzione R C, Plampin J N and Mills J E 1986 J. Org. Chem. 51 1882
Mantri P, Duffy D E and Kettner C A 1996 J. Org. Chem. 61 5690
Dempcy R O, Browne K A and Bruice T C 1995 J. Am. Chem. Soc. 117 6140
Sigman M S and Jacobsen E N 1998 J. Am. Chem. Soc. 120 4901
Whitesides G M and Ismagilov R F 1999 Science 284 89
Chigwada T R and Simoyi R H 2005 J. Phys. Chem. A. 109 1094
Gao Q Y, Liu B, Li L H and Wang J C 2007 J. Phys. Chem A. 111 872
Miller A E, Bischoff J J and Pae K 1988 Chem. Res. Toxicol. 1 169
Wiequn Z, Wen Y and L Qiu 2005 J. Mol. Struct. (THEOCHEM) 133
Peng K, Yang W and Zhou W 2009 Int. J. Quantum Chem. 109 811
Dill K A 1990 Biochemistry 29 7133
Jeffrey G A and Saenger W 1991 In Hydrogen Bonding in Biological Structures (Berlin: Springer-Verlag)
Stickle D F, Presta L G, Dill K A and Rose G D 1992 J. Mol. Biol. 226 1143
Mardyukov A, Sanchez-Garcia E, Rodziewicz P, Doltsinis N L and Sander W 2007 J. Phys. Chem. A. 111 10552
Frey J A and Leutwyler S 2006 J. Phys. Chem. A. 110 12512
Grabowski S J, Sokalski W A and Leszczynski J 2006 J. Phys. Chem. A. 110 4772
Papmokos G V and Demetropoulos I N 2004 J. Phys. Chem. A. 108 7291
Tsuchida E 2004 J. Chem. Phys 121 4740
Bende A and Suhai S 2005 Int. J. Quantum Chem. 103 841
Varga R, Garza J, Friesner R A, Stern H, Hay B P and Dixon D A 2001 J. Phys. Chem. A. 105 4963
Desfrancois C, Peiquet V, Carles S, Schermann J P and Andamowicz L 1998 Chem. Phys. 239 475
Cabaleiro-Lago E M and Otero J R 2002 J. Chem. Phys. 117 1621
Belosludov R V, Li Z and Kawazoe Y 1999 Mol. Eng. 8 105
Masunov A and Dannerberg J J 199 J. Phys. Chem. A. 103 178
Wallqvist A and Karlström G 1989 Chem. Scr. A. 29 1989
Tanaka H, Touhara H and Nakanishi K 1985 J. Chem. Phys. 82 5184
Jakli G and van Hook W W 1981 J. Phys. Chem. 85 3480
Adams R, Balyuzi H M and Burge R E 1977 J. Appl. Crystallogr. 10 256
Frisch M J, Trucks G W, Schlegel H B, Scuseria G E, Robb M A, Cheeseman J R, Scalmani G, Barone V, Mennucci B, Petersson G A, Nakatsuji H, Caricato M, Li X, Hratchian H P, Izmaylov A F, Bloino J, Zheng G, Sonnenberg J L, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery J A, Peralta J E, Ogliaro F, Bearpark M, Heyd J J, Brothers E, Kudin K N, Staroverov V N, Kobayashi R, Normand J, Raghavachari K, Rendel A, Burant J C, Iyengar S S, Tomasi J, Cossi M, Rega N, Millam J M, Klene M, Knox J E, Cross J B, Bakken V, Adamo C, Jaramillo C, Gomperts R, Stratmann R E, Yazyev O, Austin A J, Cammi R, Pomelli C, Ochterski J W, Martin R L, Morokuma K, Zakrzewski V G, Voth G A, Salvador P, Dannenberg J J, Dapprich S, Daniels A D, Farkas O, Foresman J B, Ortiz J V, Cioslowski J and Fox D J 2009 Exploring Chemistry with Electronic Structure Methods; Gaussian Inc.: Wallingford, CT
Hehre W J, Radoom L, Schleyer P V R and Pople J A 1986 In Ab Initio Molecular Orbital Theory (New York: Wiley)
Foresman J B and Frisch E 1996 In Exploring Chemistry with Electronic Structure Methods: A Guide to using Gaussian. (Pittsburg: Gaussian Inc.)
Boys S F and Bernardi F 1970 Mol. Phys. 19 553
Merrick J P, Moran D and Radom L 2007 J. Phys. Chem. A. 11683
Bader R F W 1990 In Atoms in Molecules: A Quantum Theory (Oxford: Oxford University Press)
Biegler-König F and Schönbohm J 2002 AIM 2000 version 2.0, Germany
Glendening E D and Streitwieser A 1994 J. Chem. Phys. 100 2900
Glendening E D 1996 J. Am. Chem. Soc. 118 2473
Schenter G K and Glendening E D 1996 J. Phys. Chem. 100 17152
Glendening E D, Badenhoop J K, Reed A E, Carpenter J E, Bohmann J A, Morales C M and Weinhold F 2001 Theoretical Chemistry Institute, University of Wisconsin, Madison
Weinhold F and Landis C R 2001 Chem. Edu. Res. Pract. 2 91
Schmidt M W, Baldridge K K, Boatz J, Elbert S T, Gordon M S, Jensen J H, Koseki S, Matsunaga N, Nguyen K A, Su S J, Windus T L, Dupuis M and Montgomery Jr. J A 1993 J. Comput. Chem. 141347
Foster J P and Weinhold F 1980 J. Am. Chem. Soc. 102 7211
Reed A E and Weinhold F 1983 J. Chem. Phys. 78 4066
Koch U and Popelier P L A 1995 J. Phys. Chem. 99 9747
Popelier P L A 2000 In Atoms in Molecules: An Introduction (London: Pearson Education)
Langley C H and Allinger N L 2003 J. Phys. Chem. A. 107 5208
Glendening E D 2005 J. Phys. Chem. A. 109 11936
Acknowledgement
The authors are highly thankful to University Grants Commission (UGC) for financial assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information
The optimized geometrical parameters for the adducts and corresponding monomeric units using the B3LYP and MP2 method are accessible through the supporting information tables TS1–TS22. The optimized geometrical parameters for the dimers are reported in tables TS23–TS38. The values of topological properties at BCPs characterizing the hydrogen bonds in monohydrate adduct and homodimers are reported in tables TS39–S40. The atomic charges have been evaluated using NBO analysis for all the thirty-two monohydrated adducts and homodimers reported in tables TS41–TS45. Important second order stabilization energies E(2) (kcal/mol) for the orbital interactions strengthening the formation of adduct with water and their homodimers reported in TS46–TS47. Molecular electrostatic potential (MEP) maps of the molecules and their adducts with water under investigation along with the Vmax and Vmin values from blue to red regions respectively are reported in figure S1. Supplementary information is available at www.ias.ac.in/chemsci.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
KAUR, D., KHANNA, S. Hydrogen bonding of formamide, urea, urea monoxide and their thio-analogs with water and homodimers. J Chem Sci 126, 1815–1829 (2014). https://doi.org/10.1007/s12039-014-0725-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-014-0725-6