Abstract
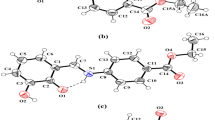
Supramolecular coordination complex (SCC) possessing spatially arranged three anthraquinone dimers in a slipped-cofacial orientation was achieved by the treatment of Re2(CO)10, 2-hydroxymethylanthraquinone and tritopic N-donor via fac-Re(CO)3-directed one pot approach. The off-set π-stacking and C≡O···H bonding interactions stabilize the ring structure.

Supramolecular coordination complex (SCC) possessing spatially arranged three anthraquinone dimers in a slipped-cofacial orientation was achieved by the treatment of Re2(CO)10, 2-hydroxymethylanthraquinone and tritopic N-donor via fac-Re(CO)3-directed one pot approach. The offset π-stacking and C≡O···H bonding interactions stabilize the ring structure.





Similar content being viewed by others
References
(a) Lehn J M 1995 In Supramolecular Chemistry, Concepts and Perspectives (Weinheim: VCH); (b) Janiak C 2000 J. Chem. Soc., Dalton Trans. 3885; (c) Chakrabarty R, Mukherjee P S and Stang P J 2011 Chem. Rev. 111 6810; (d) Fujita M, Umemoto K, Yoshizawa M, Fujita N, Kusukawa T and Biradha K 2001 Chem. Commun. 509; (e) Gianneschi N C, Masar III M S and Mirkin C A 2005 Acc. Chem. Res. 38 825; (f) Han Y F, Jia W G and Jin G X 2009 Chem. Soc. Rev. 38 3419; (g) Nohra B, Graule S, Lescop C and Reau R 2006 J. Am. Chem. Soc. 128 3520
(a) Amouri H, Desmarets C and Moussa J 2012 Chem. Rev. 112 2015; (b) Liao R T, Yang W C, Thanasekaran P, Tsai C C, Sathiyendiran M, Liu Y H, Rajendran T, Lin H M, Tseng T W and Lu K L 2008 Chem. Commun. 3175; (c) Shankar B, Elumalai P, Hussain F and Sathiyendiran M 2013 J. Organomet. Chem. 732 130; (d) Pardo E, Faus J, Lloret F, Munoz M C, Cano J, Ottenwaelder X, Journaux Y, Carrasco R, Blay G, Fernandez I and Ruiz-García R 2003 J. Am. Chem. Soc. 125 10770; (e) Shankar B, Sahu S, Deibel, N, Schweinfurth D, Sarkar B, Elumalai P, Gupta D, Hussain F, Krishnamoorthy G and Sathiyendiran M 2014 Inorg. Chem. 53 922; (f) Shankar B, Elumalai P, Shanmugam R, Singh V, Masram D T and Sathiyendiran M 2013 Inorg. Chem. 52 10217; (g) Rajakannu P, Elumalai P, Shankar B, Hussain F and Sathiyendiran M 2013 Dalton Trans. 42 11359; (h) Gupta D, Shankar B, Elumalai P, Shanmugam R, Mobin S M, Weisser F, Sarkar B and Sathiyendiran M 2014 J. Organomet. Chem. 754 59; (i) Shankar B, Rajakannu P, Kumar S, Gupta D, Kannan T and Sathiyendiran M 2011 Inorg. Chem. Commun. 14 374; (j) Rajakannu P, Elumalai P, Hussain F andSathiyendiran M 2013 J. Organomet. Chem. 725 1; (k) Shankar B, Elumalai P, Shanmugam R and Sathiyendiran M 2014 J. Organomet. Chem. 749 224; (l) Rajakannu P, Shankar B, Yadav A, Shanmugam R, Gupta D, Hussain F, Chang C H, Sathiyendiran M and Lu K L 2011 Organometallics 30 3168
(a) Yang J, Yoon M C, Yoo H, Kim P and Kim D 2012 Chem. Soc. Rev. 41 4808; (b) Ahrens M J, Sinks L E, Rybtchinski B, Liu W, Jones B A, Giaimo J M, Gusev A V, Goshe A J, Tiede D M and Wasielewski M R 2004 J. Am. Chem. Soc. 126 8284; (c) Satake A and Kobuke Y 2007 Org. Biomol. Chem. 5 1679; (d) McDermott G, Prince S M, Freer A A, Hawthornthwaite-Lawless A M, Papiz M Z, Cogdell R J and Isaacs N W 1995 Nature 374 517; (e) Bhosale S, Sisson A L, Talukdar P, Fürstenberg A, Banerji N, Vauthey E, Bollot G, Mareda J, Röger C, Würthner F, Sakai N and Matile S 2006 Science 84 313
(a) Benkstein K D and Hupp J T 2000 Mol. Cryst. Liq. Cryst. 342 151; (b) Sun S S and Lees A J 2001 Chem. Commun. 103; (c) Manimaran B, Rajendran T, Lu Y L, Lee G H, Peng S M and Lu K L 2001 Eur. J. Inorg. Chem. 633; (d) Gupta D, Rajakannu P, Shankar B, Shanmugam R, Hussain F, Sarkar B and Sathiyendiran M 2011 Dalton Trans. 40 5433; (e) Wu T, Weng L H and Jin G X 2012 Chem. Commun. 48 4435; (f) Mirtschin S, Slabon-Turski A, Scopelliti R, Velders A H and Severin K 2010 J. Am. Chem. Soc. 132 14004; (g) Therrien B 2009 Eur. J. Inorg. Chem. 2445; (h) Wu J Y, Chang C H, Thanasekaran P, Tsai C C, Tseng T W, Lee G H, Peng S M and Lu K L 2008 Dalton Trans. 6110
Song Z, Zhan H and Zhou Y 2009 Chem. Commun. 448
(a) Desiraju G R 2005 Chem. Commun. 2995; (b) Rajadurai C, Fuhr O, Enkelmann V and Baumgarten M 2006 J. Phys. Org. Chem. 19 257
Bailar Jr. J C 1958 J. Inorg. Nucl. Chem. 8 165
(a) Govindaswamy P, Linder D, Lacour J, Süss-Fink G and Therrien B 2006 Chem. Commun. 4691; (b) Caskey D C, Yamamoto T, Addicott C, Shoemaker R K, Vacek J, Hawkridge A M, Muddiman D C, Kottas G S, Michl J and Stang P J 2008 J. Am. Chem. Soc. 130 7620
(a) Pawin G, Solanki U, Kwon K Y, Wong K L, Lin X, Jiao T and Bartels L 2007 J. Am. Chem. Soc. 129 12056; (b) Pawin G, Wong K L, Kwon K Y and Bartels L 2006 Science 313 961
(a) CrysAlis Pro software system, Version 171.32; Oxford Diffraction Ltd., Oxford, U.K., 2007; (b) Sheldrick G M 2008 Acta. Crystallogr. A 64 112
Acknowledgements
We thank Prof. R. Murugavel, IIT-Bombay for scientific support and the University Grants Commission (UGC) (F. No. 41-218/2012 (SR)), India for financial support. DG and BS thank the Council of Scientific and Industrial Research (CSIR) for Senior Research Fellowship. We are grateful to Perkin-Elmer for ESI-Mass analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information
The electronic supplementary information can be seen at www.ias.ac.in/chemsci.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
GUPTA, D., RAJAKANNU, P., SHANKAR, B. et al. Synthesis and crystal structure of a wheel-shaped supramolecular coordination complex. J Chem Sci 126, 1501–1506 (2014). https://doi.org/10.1007/s12039-014-0655-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-014-0655-3