Abstract
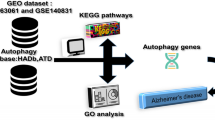
This study aimed to identify autophagy-related candidate genes for the early diagnosis of Alzheimer’s disease (AD) and elucidate their potential molecular mechanisms. Differentially expressed genes (DEGs) and phenotype-associated significant module genes were obtained using the “limma” package and weighted gene co-expression network analysis (WGCNA) based on hippocampal tissue datasets from AD patients and control samples. The intersection between the list of autophagy-related genes (ATGs), DEGs, and module genes was further investigated to obtain AD-autophagy-related differential expression genes (ATDEGs). Subsequently, the least absolute shrinkage and selection operator (LASSO) algorithm was utilized to identify hub genes, and a second intersection was performed with important module genes from the protein–protein interaction (PPI) network to obtain co-hub genes. Finally, a diagnostic model was constructed by receiver operating characteristic (ROC) analysis to determine the candidate genes with high diagnostic efficacy in the external validation set. Moreover, immune infiltration analysis was performed on AD patient brain tissues and explore the correlation between candidate genes and immune cells. We further analyzed the expression level of candidate genes in the SH-SY5Y cells with Aβ25–35 (25 µM). Among the 17 identified AD-ATDEGs, ATP6V1E1 stood out with area under the curve (AUC) values of 0.869, 0.817, and 0.714 in the external validation set, underscoring its high diagnostic efficacy in both hippocampal and peripheral blood contexts for AD patients. Meanwhile, ATP6V1E1 expression was positively correlated with effector memory CD4 + T cells, while negatively correlated with natural killer T cells and activated CD4 + T cells. Results from quantitative PCR (qPCR) and immunofluorescence assays indicated a reduction in ATP6V1E1 expression, aligning with our database analysis findings. In summary, ATP6V1E1 as a candidate gene provides a new perspective for the early identification and pathogenesis of AD.








Similar content being viewed by others
Data Availability
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
References
Crous-Bou M, Minguillón C, Gramunt N, Molinuevo JL (2017) Alzheimer’s disease prevention: from risk factors to early intervention. Alzheimer’s Res Ther 9(1):71. https://doi.org/10.1186/s13195-017-0297-z
Scheltens P, De Strooper B, Kivipelto M, Holstege H, Chételat G, Teunissen CE, Cummings J, van der Flier WM (2021) Alzheimer’s disease. Lancet (London, England) 397(10284):1577–1590. https://doi.org/10.1016/S0140-6736(20)32205-4
Nasrallah IM, Wolk DA (2014) Multimodality imaging of Alzheimer disease and other neurodegenerative dementias. J Nucl Med 55(12):2003–2011. https://doi.org/10.2967/jnumed.114.141416
Selkoe DJ (2001) Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev 81(2):741–766. https://doi.org/10.1152/physrev.2001.81.2.741
Zhang XX, Tian Y, Wang ZT, Ma YH, Tan L, Yu JT (2021) The epidemiology of Alzheimer’s disease modifiable risk factors and prevention. J Prev Alzheimer’s Dis 8(3):313–321. https://doi.org/10.14283/jpad.2021.15
Li M, Zeng Y, Huang Z, Zhang L, Liu Y (2023) Vertical graphene-based printed electrochemical biosensor for simultaneous detection of four Alzheimer’s disease blood biomarkers. Biosensors 13(8):758. https://doi.org/10.3390/bios13080758
Zhang Z, Yang X, Song YQ, Tu J (2021) Autophagy in Alzheimer’s disease pathogenesis: therapeutic potential and future perspectives. Ageing Res Rev 72:101464. https://doi.org/10.1016/j.arr.2021.101464
Li Q, Liu Y, Sun M (2017) Autophagy and Alzheimer’s disease. Cell Mol Neurobiol 37(3):377–388. https://doi.org/10.1007/s10571-016-0386-8
Lee JH, Yang DS, Goulbourne CN, Im E, Stavrides P, Pensalfini A, Chan H, Bouchet-Marquis C, Bleiwas C, Berg MJ, Huo C, Peddy J, Pawlik M, Levy E, Rao M, Staufenbiel M, Nixon RA (2022) Faulty autolysosome acidification in Alzheimer’s disease mouse models induces autophagic build-up of Aβ in neurons, yielding senile plaques. Nat Neurosci 25(6):688–701. https://doi.org/10.1038/s41593-022-01084-8
Tran M, Reddy PH (2021) Defective autophagy and mitophagy in aging and Alzheimer’s disease. Front Neurosci 14:612757. https://doi.org/10.3389/fnins.2020.612757
Yu WH, Cuervo AM, Kumar A, Peterhoff CM, Schmidt SD, Lee JH, Mohan PS, Mercken M, Farmery MR, Tjernberg LO, Jiang Y, Duff K, Uchiyama Y, Näslund J, Mathews PM, Cataldo AM, Nixon RA (2005) Macroautophagy–a novel beta-amyloid peptide-generating pathway activated in Alzheimer’s disease. J Cell Biol 171(1):87–98. https://doi.org/10.1083/jcb.200505082
Zheng X, Lin W, Jiang Y, Lu K, Wei W, Huo Q, Cui S, Yang X, Li M, Xu N, Tang C, Song JX (2021) Electroacupuncture ameliorates beta-amyloid pathology and cognitive impairment in Alzheimer disease via a novel mechanism involving activation of TFEB (transcription factor EB). Autophagy 17(11):3833–3847. https://doi.org/10.1080/15548627.2021.1886720
Canchi S, Raao B, Masliah D, Rosenthal SB, Sasik R, Fisch KM, De Jager PL, Bennett DA, Rissman RA (2019) Integrating gene and protein expression reveals perturbed functional networks in Alzheimer’s disease. Cell Rep 28(4):1103-1116.e4. https://doi.org/10.1016/j.celrep.2019.06.073
Heckmann BL, Teubner BJW, Tummers B, Boada-Romero E, Harris L, Yang M, Guy CS, Zakharenko SS, Green DR (2019) LC3-associated endocytosis facilitates β-amyloid clearance and mitigates neurodegeneration in murine Alzheimer’s disease. Cell 178(3):536-551.e14. https://doi.org/10.1016/j.cell.2019.05.056
Jefferies KC, Forgac M (2008) Subunit H of the vacuolar (H+) ATPase inhibits ATP hydrolysis by the free V1 domain by interaction with the rotary subunit F. J Biol Chem 283(8):4512–4519. https://doi.org/10.1074/jbc.M707144200
Levine B, Mizushima N, Virgin HW (2011) Autophagy in immunity and inflammation. Nature 469(7330):323–335. https://doi.org/10.1038/nature09782
Wu KM, Zhang YR, Huang YY, Dong Q, Tan L, Yu JT (2021) The role of the immune system in Alzheimer’s disease. Ageing Res Rev 70:101409. https://doi.org/10.1016/j.arr.2021.101409
McLarnon JG (2021) A leaky blood-brain barrier to fibrinogen contributes to oxidative damage in Alzheimer’s disease. Antioxidants (Basel, Switzerland) 11(1):102. https://doi.org/10.3390/antiox11010102
Butler R, Bradford D, Rodgers KE (2022) Analysis of shared underlying mechanism in neurodegenerative disease. Front Aging Neurosci 14:1006089. https://doi.org/10.3389/fnagi.2022.1006089
Liang P, Le W (2015) Role of autophagy in the pathogenesis of multiple sclerosis. Neurosci Bull 31(4):435–444. https://doi.org/10.1007/s12264-015-1545-5
Deretic V, Saitoh T, Akira S (2013) Autophagy in infection, inflammation and immunity. Nat Rev Immunol 13(10):722–737. https://doi.org/10.1038/nri3532
Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez JC, Müller M (2011) pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics 12:77. https://doi.org/10.1186/1471-2105-12-77
Hughes TR, Lambert SA (2017) Transcription factors read epigenetics. Science 356(6337):489–490. https://doi.org/10.1126/science.aan2927
Lambert SA, Jolma A, Campitelli LF, Das PK, Yin Y, Albu M, Chen X, Taipale J, Hughes TR, Weirauch MT (2018) The human transcription factors. Cell 172(4):650–665. https://doi.org/10.1016/j.cell.2018.01.029
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP (2005) Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 102(43):15545–15550. https://doi.org/10.1073/pnas.0506580102
Kontos E, Blake KD, Chou WY, Prestin A (2014) Predictors of eHealth usage: insights on the digital divide from the Health Information National Trends Survey 2012. J Med Internet Res 16(7):e172. https://doi.org/10.2196/jmir.3117
Selkoe DJ, Hardy J (2016) The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med 8(6):595–608. https://doi.org/10.15252/emmm.201606210
Linda K, Lewerissa EI, Verboven AHA, Gabriele M, Frega M, Klein Gunnewiek TM, Devilee L, Ulferts E, Hommersom M, Oudakker A, Schoenmaker C, van Bokhoven H, Schubert D, Testa G, Koolen DA, de Vries BBA, Nadif Kasri N (2022) Imbalanced autophagy causes synaptic deficits in a human model for neurodevelopmental disorders. Autophagy 18(2):423–442. https://doi.org/10.1080/15548627.2021.1936777
Liang Y (2019) Emerging concepts and functions of autophagy as a regulator of synaptic components and plasticity. Cells 8(1):34. https://doi.org/10.3390/cells8010034
Blennow K, Zetterberg H (2018) Biomarkers for Alzheimer’s disease: current status and prospects for the future. J Intern Med 284(6):643–663. https://doi.org/10.1111/joim.12816
Qu Y, Ma YH, Huang YY, Ou YN, Shen XN, Chen SD, Dong Q, Tan L, Yu JT (2021) Blood biomarkers for the diagnosis of amnestic mild cognitive impairment and Alzheimer’s disease: a systematic review and meta-analysis. Neurosci Biobehav Rev 128:479–486. https://doi.org/10.1016/j.neubiorev.2021.07.007
Miyoshi E, Morabito S, Swarup V (2021) Systems biology approaches to unravel the molecular and genetic architecture of Alzheimer’s disease and related tauopathies. Neurobiol Dis 160:105530. https://doi.org/10.1016/j.nbd.2021.105530
Adewale Q, Khan AF, Carbonell F, Iturria-Medina Y, Alzheimer’s Disease Neuroimaging Initiative, (2021) Integrated transcriptomic and neuroimaging brain model decodes biological mechanisms in aging and Alzheimer’s disease. eLife 10:e62589. https://doi.org/10.7554/eLife.62589
Liang WS, Reiman EM, Valla J, Dunckley T, Beach TG, Grover A, Niedzielko TL, Schneider LE, Mastroeni D, Caselli R, Kukull W, Morris JC, Hulette CM, Schmechel D, Rogers J, Stephan DA (2008) Alzheimer’s disease is associated with reduced expression of energy metabolism genes in posterior cingulate neurons. Proc Natl Acad Sci USA 105(11):4441–4446. https://doi.org/10.1073/pnas.0709259105
Vella D, Marini S, Vitali F, Di Silvestre D, Mauri G, Bellazzi R (2018) MTGO: PPI network analysis via topological and functional module identification. Sci Rep 8(1):5499. https://doi.org/10.1038/s41598-018-23672-0
Yu H, Wang F, Wu JJ, Gong J, Bi S, Mao Y, Jia D, Chai GS (2023) Integrated transcriptomics reveals the brain and blood biomarkers in Alzheimer’s disease. CNS Neurosci Ther 29(12):3943–3951. https://doi.org/10.1111/cns.14316
Barrett T, Wilhite SE, Ledoux P, Evangelista C, Kim IF, Tomashevsky M, Marshall KA, Phillippy KH, Sherman PM, Holko M, Yefanov A, Lee H, Zhang N, Robertson CL, Serova N, Davis S, Soboleva A (2013) NCBI GEO: archive for functional genomics data sets–update. Nucleic Acids Res 41:D991–D995. https://doi.org/10.1093/nar/gks1193
Jiang S, Bhaskar K (2020) Degradation and transmission of tau by autophagic-endolysosomal networks and potential therapeutic targets for tauopathy. Front Mol Neurosci 13:586731. https://doi.org/10.3389/fnmol.2020.586731
Ferragut Cardoso AP, Banerjee M, Nail AN, Lykoudi A, States JC (2021) miRNA dysregulation is an emerging modulator of genomic instability. Semin Cancer Biol 76:120–131. https://doi.org/10.1016/j.semcancer.2021.05.004
Wang Y, Cui Y, Liu J, Song Q, Cao M, Hou Y, Zhang X, Wang P (2022) Krüppel-like factor 5 accelerates the pathogenesis of Alzheimer’s disease via BACE1-mediated APP processing. Alzheimer’s Res Ther 14(1):103. https://doi.org/10.1186/s13195-022-01050-3
Zhou Y, Ge Y, Liu Q, Li YX, Chao X, Guan JJ, Diwu YC, Zhang Q (2021) LncRNA BACE1-AS promotes autophagy-mediated neuronal damage through the miR-214-3p/ATG5 signalling axis in Alzheimer’s disease. Neuroscience 455:52–64. https://doi.org/10.1016/j.neuroscience.2020.10.028
Silva JD, Taglialatela G, Jupiter DC (2023) Reduced prevalence of dementia in patients prescribed tacrolimus, sirolimus, or cyclosporine. J Alzheimer’s Dis 95(2):585–597. https://doi.org/10.3233/JAD-230526
Maltsev AV, Nikiforova AB, Bal NV, Balaban PM (2022) Amyloid Aβ25-35 aggregates say ‘NO’ to long-term potentiation in the hippocampus through activation of stress-induced phosphatase 1 and mitochondrial Na+/Ca2+ exchanger. Int J Mol Sci 23(19):11848. https://doi.org/10.3390/ijms231911848
Liu C, Xu S, Liu Q, Chai H, Luo Y, Li S (2023) Identification of immune cells infiltrating in hippocampus and key genes associated with Alzheimer’s disease. BMC Med Genomics 16(1):53. https://doi.org/10.1186/s12920-023-01458-2
Huang X, Reynolds AD, Mosley RL, Gendelman HE (2009) CD 4+ T cells in the pathobiology of neurodegenerative disorders. J Neuroimmunol 211(1–2):3–15. https://doi.org/10.1016/j.jneuroim.2009.04.006
Lai Y, Lin P, Lin F, Chen M, Lin C, Lin X, Wu L, Zheng M, Chen J (2022) Identification of immune microenvironment subtypes and signature genes for Alzheimer’s disease diagnosis and risk prediction based on explainable machine learning. Front Immunol 13:1046410. https://doi.org/10.3389/fimmu.2022.1046410
Zhang Y, Fung ITH, Sankar P, Chen X, Robison LS, Ye L, D’Souza SS, Salinero AE, Kuentzel ML, Chittur SV, Zhang W, Zuloaga KL, Yang Q (2020) Depletion of NK cells improves cognitive function in the Alzheimer disease mouse model. J Immunol 205(2):502–510. https://doi.org/10.4049/jimmunol.2000037
Acknowledgements
We acknowledge all GEO data builders and data contributors. We thank the Xiangya Hospital provides SH-SY5Y cells.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design, and Jian Wang and Xinhua Huo contributed equally to this work. Material preparation, data collection, and analysis were performed by Xinhua Huo and Huiqin Zhou. The first draft of the manuscript was written by Jian Wang and Xinhua Huo. Huasheng Liu and Na Lu participated in the revision of the manuscript. Xinhua Huo, Xiaofeng Li, and Xuan Sun performed the cell culture, data correction, and image rendering. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, J., Huo, X., Zhou, H. et al. Identification of Autophagy-Related Candidate Genes in the Early Diagnosis of Alzheimer’s Disease and Exploration of Potential Molecular Mechanisms. Mol Neurobiol (2024). https://doi.org/10.1007/s12035-024-04011-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12035-024-04011-z