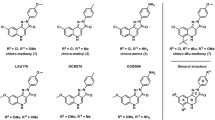
Abstract
In the 1980s, the identification of specific pharmacological antagonists played a crucial role in enhancing our comprehension of the physiological mechanisms associated with α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptors (AMPARs). The primary objective of this investigation was to identify specific AMPA receptor antagonists, namely 2,3-benzodiazepines, that function as negative allosteric modulators (NAMs) at distinct locations apart from the glutamate recognition site. These compounds have exhibited a diverse array of anticonvulsant properties. In order to conduct a more comprehensive investigation, the study utilized whole-cell patch-clamp electrophysiology to analyze the inhibitory effect and selectivity of benzodiazepine derivatives that incorporate coumarin rings in relation to AMPA receptors. The study’s main objective was to acquire knowledge about the relationship between the structure and activity of the compound and comprehend the potential effects of altering the side chains on negative allosteric modulation. The investigation provided crucial insights into the interaction between eight CD compounds and AMPA receptor subunits. Although all compounds demonstrated effective blockade, CD8 demonstrated the greatest potency and selectivity towards AMPA receptor subunits. The deactivation and desensitization rates were significantly influenced by CD8, CD6, and CD5, distinguishing them from the remaining five chemicals. The differences in binding and inhibition of AMPA receptor subunits can be attributed to structural discrepancies among the compounds. The carboxyl group of CD8, situated at the para position of the phenyl ring, substantially influenced the augmentation of AMPA receptor affinity. The findings of this study highlight the potential of pharmaceutical compounds that specifically target AMPA receptors to facilitate negative allosteric modulation.
Graphical Abstract







Similar content being viewed by others
Data Availability
Data collected or analyzed in this investigation are included in this manuscript and its supplemental material file.
Abbreviations
- CD:
-
coumarin-benzodiazepine hybrid compounds
- iGluRs:
-
ionotropic glutamate receptors
- AMPA:
-
α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid
- NMDA:
-
N-methyl-D-aspartate
- HEK293:
-
human embryonic kidney 293 cells
- AMPARs:
-
AMPA receptors
- CNS:
-
central nervous system
- NTD:
-
N-terminal extracellular amino domain
- LBD:
-
ligand binding domain
- TMD:
-
transmembrane domain
- LTP:
-
long-term potentiation
- LTD:
-
long-term depression
- NAM:
-
negative allosteric modulator
- PAM:
-
positive allosteric modulator
- GFP:
-
green fluorescent protein
- DMF:
-
N, N-dimethylformamide
- ANOVA:
-
a one-way analysis of variance
References
Coombs ID, Cull-Candy SG (2021) Single-channel mechanisms underlying the function, diversity and plasticity of AMPA receptors. Neuropharmacology 198:108781
Greger IH, Watson JF, Cull-candy SG (2017) structural and functional architecture of AMPA-type glutamate receptors and their auxiliary proteins. Neuron 94(4):713–730
Nakagawa T et al (2005) Structure and different conformational states of native AMPA receptor complexes. Nature 433(7025):545–549
Chen S, Gouaux E (2019) Structure and mechanism of AMPA receptor - auxiliary protein complexes. Curr Opin Struct Biol 54:104–111
Diering GH, Huganir RL (2018) The AMPA receptor code of synaptic plasticity. Neuron 100(2):314–329
Sprengel R (2006) Role of AMPA receptors in synaptic plasticity. Cell Tissue Res 326(2):447–455
Zhao Y et al (2019) Architecture and subunit arrangement of native AMPA receptors elucidated by cryo-EM. Science 364(6438):355–362
Henley JM, Wilkinson KA (2016) Synaptic AMPA receptor composition in development, plasticity and disease. Nat Rev Neurosci 17(6):337–350
Salpietro V et al (2019) AMPA receptor GluA2 subunit defects are a cause of neurodevelopmental disorders. Nat Commun 10(1):3094
Henley JM et al (2021) Kainate and AMPA receptors in epilepsy: cell biology, signalling pathways and possible crosstalk. Neuropharmacology 195:108569
Wright A, Vissel B (2012) The essential role of AMPA receptor GluR2 subunit RNA editing in the normal and diseased brain. Front Mol Neurosci 5:34
Cull-Candy SG, Farrant M (2021) Ca2+-permeable AMPA receptors and their auxiliary subunits in synaptic plasticity and disease. Physiol J 599(10):2655–2671
Rogawski MAJANS (2013) AMPA receptors as a molecular target in epilepsy therapy. Acta Neurol Scand 127:9–18
Anwar H et al (2020) Epileptic seizures. Discoveries (Craiova) 8(2):e110
Balannik V et al (2005) Molecular mechanism of AMPA receptor non-competitive antagonism. Neuron 48(2):279–288
Vizi ES, Mike A, Tarnawa I (1996) 2,3-Benzodiazepines (GYKI 52466 and Analogs): negative allosteric modulators of AMPA receptors. CNS Drug Rev 2(1):91–126
Howes JF, Bell C (2007) Talampanel. Neurotherapeutics 4(1):126–129
Langan YM et al (2003) Talampanel, a new antiepileptic drug: single- and multiple-dose pharmacokinetics and initial 1-week experience in patients with chronic intractable epilepsy. Epilepsia 44(1):46–53
Satlin A, Kramer LD, Laurenza A (2013) Development of perampanel in epilepsy. Acta Neurol Scand Suppl 197:3–8
Franco V et al (2013) Novel treatment options for epilepsy: focus on perampanel. Pharmacol Res 70(1):35–40
Trinka E et al (2016) Perampanel for focal epilepsy: insights from early clinical experience. Acta Neurol Scand 133(3):160–172
Jaradat N et al (2022) The effect of novel negative allosteric 2,3-benzodiazepine on glutamate AMPA receptor and their cytotoxicity. J Mol Struct 1261:132936
Qneibi M et al (2020) Ortho versus meta chlorophenyl-2,3-benzodiazepine analogues: synthesis, molecular modeling, and biological activity as AMPAR antagonists. ACS Omega 5(7):3588–3595
Qneibi M et al (2022) Affecting AMPA receptor biophysical gating properties with negative allosteric modulators. Mol Neurobiol 59(9):5264–5275
Qneibi M et al (2022) α-Lipoic acid derivatives as allosteric modulators for targeting AMPA-type glutamate receptors’ gating modules. Cells 11(22):3608
Yuan CL et al (2019) Modulation of AMPA receptor gating by the anticonvulsant drug, perampanel. ACS Med Chem Lett 10(3):237–242
Augustin K et al (2018) Perampanel and decanoic acid show synergistic action against AMPA receptors and seizures. Epilepsia 59(11):e172–e178
Taniguchi S, Stolz JR, Swanson GT (2022) The antiseizure drug perampanel is a subunit-selective negative allosteric modulator of kainate receptors. J Neurosci 42(28):5499–5509
Stenum-Berg C et al (2019) Mutational analysis and modeling of negative allosteric modulator binding sites in AMPA receptors. Mol Pharmacol 96(6):835–850
Menniti FS et al (2000) characterization of the binding site for a novel class of non-competitive α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor antagonists. Mol Pharmacol 58(6):1310–1317
Sólyom S, Tarnawa I (2002) Non-competitive AMPA antagonists of 2, 3-benzodiazepine type. Curr Pharm Des 8(10):913–939
Mittapalli GK, Roberts E (2014) Structure activity relationships of novel antiepileptic drugs. Curr Med Chem 21(6):722–754
Qneibi M et al (2021) The AMPA receptor biophysical gating properties and binding site: focus on novel curcumin-based diazepines as non-competitive antagonists. Bioorg Chem 116:105406
Gümüş M (2021) Synthesis and characterization of novel hybrid compounds containing coumarin and benzodiazepine rings based on dye. J Heterocycl Chem 58(10):1943–1954
Qneibi M et al (2019) inhibition and assessment of the biophysical gating properties of GluA2 and GluA2/A3 AMPA receptors using curcumin derivatives. Plos one 14(8):e0221132
Qneibi M et al (2019) The inhibitory role of curcumin derivatives on AMPA receptor subunits and their effect on the gating biophysical properties. Eur J Pharm Sci 136:104951
Li G, Pei W, Niu L (2003) Channel-opening kinetics of GluR2Q(flip) AMPA receptor: a laser-pulse photolysis study. Biochemistry 42(42):12358–12366
Huang Z et al (2010) Potent and selective inhibition of the open-channel conformation of AMPA receptors by an RNA aptamer. Biochemistry 49(27):5790–5798
Emnett CM et al (2013) Indistinguishable synaptic pharmacodynamics of the N-methyl-D-aspartate receptor channel blockers memantine and ketamine. Mol Pharmacol 84(6):935–947
Jaradat N et al (2022) Assessing Artemisia arborescens essential oil compositions, antimicrobial, cytotoxic, anti-inflammatory, and neuroprotective effects gathered from two geographic locations in Palestine. Ind Crops Prod 176:114360
Qneibi M, Jaradat N, Emwas N (2019) Effect of geraniol and citronellol essential oils on the biophysical gating properties of AMPA receptors. Appl Sci 9(21):4693
Qneibi M et al (2022) Targeting the kinetics mechanism of AMPA receptor inhibition by 2-oxo-3H-benzoxazole derivatives. Bioorg Chem 129:106163
Narangoda C, Sakipov SN, Kurnikova MG (2019) AMPA receptor non-competitive inhibitors occupy a promiscuous binding site. ACS Chem Neurosci 10(11):4511–4521
Krintel C et al (2021) binding of a negative allosteric modulator and competitive antagonist can occur simultaneously at the ionotropic glutamate receptor GluA2. Febs J 288(3):995–1007
Szymańska E et al (2017) Pharmacological characterization and binding modes of novel racemic and optically active phenylalanine-based antagonists of AMPA receptors. Eur J Med Chem 138:874–883
Armstrong N, Gouaux E (2000) Mechanisms for activation and antagonism of an AMPA-sensitive glutamate receptor: crystal structures of the GluR2 ligand binding core. Neuron 28(1):165–181
Yelshanskaya MV et al (2016) Structural bases of non-competitive inhibition of AMPA-subtype ionotropic glutamate receptors by antiepileptic drugs. Neuron 91(6):1305–1315
Bleakman D et al (1996) activity of 2,3-benzodiazepines at native rat and recombinant human glutamate receptors in vitro: stereospecificity and selectivity profiles. Neuropharmacology 35(12):1689–1702
Acknowledgements
The authors are grateful to An-Najah National University (www.najah.edu) for its support in this research.
Author information
Authors and Affiliations
Contributions
Mohammad Qneibi: conceptualization, methodology, validation, investigation, writing—original draft, manuscript drafting, data curation, and project. Mohammed Hawash: validation, data curation, and manuscript drafting. Mehmet Gümüş: synthesis. İrfan Çapan: validation. Yusuf Sert: validation. Sosana Bdir: validation and manuscript drafting. İrfan Koca: Synthesis and manuscript drafting. Mohammad Bdair: manuscript drafting.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
All authors have given their consent for publication.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qneibi, M., Hawash, M., Gümüş, M. et al. Deciphering the Biophysical Properties of Ion Channel Gating Pores by Coumarin–Benzodiazepine Hybrid Derivatives: Selective AMPA Receptor Antagonists. Mol Neurobiol (2023). https://doi.org/10.1007/s12035-023-03871-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12035-023-03871-1