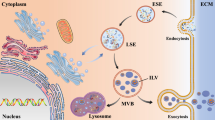
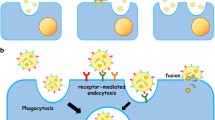
Abstract
Exosomes are small extracellular vesicles with a complex lipid-bilayer surface and 30–150 nm diameter. These vesicles play a critical role in intercellular signaling networks during physiopathological processes through data trafficking and cell reprogramming. It has been demonstrated that exosomes are involved in a variety of central nervous system (CNS) disorders such as multiple sclerosis (MS). Exosome mediators’ cell-to-cell communication is possibly by delivering their contents such as proteins, RNAs (coding and non-coding), DNAs (mitochondrial and genomic), and transposable elements to the target cells. Exosomal microRNAs (miRNAs) differ in their expression patterns in MS disease, thereby providing novel diagnostic and prognostic biomarkers and therapeutic options for better treatment of MS disease. Furthermore, these microvesicles are non-immunogenic and non-toxic therapeutic tools for transferring miRNAs across the blood-brain barrier (BBB). Collectively, exosomes could be used as novel drug delivery devices for the treatment of MS patients. This review summarized research regarding the exosomes from serum, plasma, PBMC, and other cells in MS patients and experimental models. We also provide a critical view of exosome content-mediated signaling pathways in MS, including TNF-α, TGF-β, NF-κB, and Wnt pathways. The use of exosomes as a therapeutic potential in MS has also been discussed.

Similar content being viewed by others
Data Availability
Not applicable.
References
Reich DS, Lucchinetti CF, Calabresi PA (2018) Multiple sclerosis. N Engl J Med 378:169–180. https://doi.org/10.1056/NEJMra1401483
Dolati S, Babaloo Z, Jadidi-Niaragh F et al (2017) Multiple sclerosis: therapeutic applications of advancing drug delivery systems. Biomed Pharmacother 86:343–353. https://doi.org/10.1016/j.biopha.2016.12.010
Baulina N, Kulakova O, Kiselev I et al (2018) Immune-related miRNA expression patterns in peripheral blood mononuclear cells differ in multiple sclerosis relapse and remission. J Neuroimmunol 317:67–76. https://doi.org/10.1016/j.jneuroim.2018.01.005
Bjornevik K, Cortese M, Healy BC et al (2022) Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science (1979) 375. https://doi.org/10.1126/science.abj8222
Robinson WHSL (2022) Epstein-Barr virus and multiple sclerosis. Science (1979) 375:264–265
Tacchino A, Brichetto G, Zaratin P et al (2019) Self-assessment reliability in multiple sclerosis: the role of socio-demographic, clinical, and quality of life aspects. Neurol Sci 40:617–620. https://doi.org/10.1007/s10072-018-3589-6
Chen BY, Ghezzi C, Villegas B et al (2020) 18F-FAC PET visualizes brain-infiltrating leukocytes in a mouse model of multiple sclerosis. J Nucl Med 61:757–763. https://doi.org/10.2967/jnumed.119.229351
Thompson AJ, Banwell BL, Barkhof F et al (2018) Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 17:162–173. https://doi.org/10.1016/S1474-4422(17)30470-2
Jan A, Rahman S, Khan S et al (2019) Biology, pathophysiological role, and clinical implications of exosomes: a critical appraisal. Cells 8:99. https://doi.org/10.3390/cells8020099
Azimi M, Ghabaee M, Moghadasi AN et al (2018) Immunomodulatory function of Treg-derived exosomes is impaired in patients with relapsing-remitting multiple sclerosis. Immunol Res 66:513–520. https://doi.org/10.1007/s12026-018-9008-5
Selmaj I, Mycko MP, Raine CS, Selmaj KW (2017) The role of exosomes in CNS inflammation and their involvement in multiple sclerosis. J Neuroimmunol 306:1–10. https://doi.org/10.1016/j.jneuroim.2017.02.002
Tkach M, Théry C (2016) Communication by extracellular vesicles: where we are and where we need to go. Cell 164:1226–1232. https://doi.org/10.1016/j.cell.2016.01.043
Das CK, Jena BC, Banerjee I et al (2019) Exosome as a novel shuttle for delivery of therapeutics across biological barriers. Mol Pharm 16:24–40. https://doi.org/10.1021/acs.molpharmaceut.8b00901
Salarpour S, Barani M, Pardakhty A et al (2022) The application of exosomes and exosome-nanoparticle in treating brain disorders. J Mol Liq 350:118549. https://doi.org/10.1016/J.MOLLIQ.2022.118549
Mittal R, Bencie N, Langlie J et al (2021) Exosomes as drug delivery vehicles and biomarkers for neurological and auditory systems. J Cell Physiol 236:8035–8049. https://doi.org/10.1002/jcp.30484
Zhang M, Zang X, Wang M et al (2019) Exosome-based nanocarriers as bio-inspired and versatile vehicles for drug delivery: recent advances and challenges. J Mater Chem B 7:2421–2433. https://doi.org/10.1039/C9TB00170K
Farooqi AA, Desai NN, Qureshi MZ et al (2018) Exosome biogenesis, bioactivities and functions as new delivery systems of natural compounds. Biotechnol Adv 36:328–334. https://doi.org/10.1016/j.biotechadv.2017.12.010
Rezaie J, Ajezi S, Avci ÇB et al (2018) Exosomes and their application in biomedical field: difficulties and advantages. Mol Neurobiol 55:3372–3393. https://doi.org/10.1007/s12035-017-0582-7
Terstappen GC, Meyer AH, Bell RD, Zhang W (2021) Strategies for delivering therapeutics across the blood–brain barrier. Nat Rev Drug Discov 20:362–383. https://doi.org/10.3390/pharmaceutics14050987
van Niel G, D’Angelo G, Raposo G (2018) Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol 19:213–228. https://doi.org/10.1038/nrm.2017.125
Sweeney MD, Sagare AP, Zlokovic BV (2018) Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol 14:133–150. https://doi.org/10.1038/nrneurol.2017.188
Manna I, Iaccino E, Dattilo V et al (2018) Exosome-associated miRNA profile as a prognostic tool for therapy response monitoring in multiple sclerosis patients. FASEB J 32:4241–4246. https://doi.org/10.1096/fj.201701533R
Ghini F, Rubolino C, Climent M et al (2018) Endogenous transcripts control miRNA levels and activity in mammalian cells by target-directed miRNA degradation. Nat Commun 9:3119. https://doi.org/10.1038/s41467-018-05182-9
Hosseini HM, Fooladi AAI, Nourani MR, Ghanezadeh F (2013) The role of exosomes in infectious diseases. Inflamm Allergy Drug Targets 12:29–37. https://doi.org/10.2174/1871528111312010005
Hessvik NP, Llorente A (2018) Current knowledge on exosome biogenesis and release. Cell Mol Life Sci 75:193–208. https://doi.org/10.1007/s00018-017-2595-9
Villarroya-Beltri C, Baixauli F, Mittelbrunn M et al (2016) ISGylation controls exosome secretion by promoting lysosomal degradation of MVB proteins. Nat Commun 7:13588. https://doi.org/10.1038/ncomms13588
Kahroba H, Hejazi MS, Samadi N (2019) Exosomes: from carcinogenesis and metastasis to diagnosis and treatment of gastric cancer. Cell Mol Life Sci 76:1747–1758. https://doi.org/10.1007/s00018-019-03035-2
Saeedi S, Israel S, Nagy C, Turecki G (2019) The emerging role of exosomes in mental disorders. Transl Psychiatry 9:122. https://doi.org/10.1038/s41398-019-0459-9
Horibe S, Tanahashi T, Kawauchi S et al (2018) Mechanism of recipient cell-dependent differences in exosome uptake. BMC Cancer 18:47. https://doi.org/10.1186/s12885-017-3958-1
Li X, Wang Y, Wang Q et al (2018) Exosomes in cancer: small transporters with big functions. Cancer Lett 435:55–65. https://doi.org/10.1016/j.canlet.2018.07.037
Tran TH, Mattheolabakis G, Aldawsari H, Amiji M (2015) Exosomes as nanocarriers for immunotherapy of cancer and inflammatory diseases. Clin Immunol 160:46–58. https://doi.org/10.1016/j.clim.2015.03.021
Laso-García F, Ramos-Cejudo J, Carrillo-Salinas FJ et al (2018) Therapeutic potential of extracellular vesicles derived from human mesenchymal stem cells in a model of progressive multiple sclerosis. PLoS ONE 13:e0202590. https://doi.org/10.1371/journal.pone.0202590
de Toro J, Herschlik L, Waldner C, Mongini C (2015) Emerging roles of exosomes in normal and pathological conditions: new insights for diagnosis and therapeutic applications. Front Immunol 6. https://doi.org/10.3389/fimmu.2015.00203
Kahroba H, Samadi N, Mostafazadeh M et al (2021) Evaluating the presence of deregulated tumoral onco-microRNAs in serum-derived exosomes of gastric cancer patients as noninvasive diagnostic biomarkers. BioImpacts 11. https://doi.org/10.34172/bi.2021.22178
Ebrahimkhani S, Beadnall HN, Wang C et al (2020) Serum exosome microRNAs predict multiple sclerosis disease activity after fingolimod treatment. Mol Neurobiol 57:1245–1258. https://doi.org/10.1007/s12035-019-01792-6
Record M, Subra C, Silvente-Poirot S, Poirot M (2011) Exosomes as intercellular signalosomes and pharmacological effectors. Biochem Pharmacol 81:1171–1182. https://doi.org/10.1016/j.bcp.2011.02.011
Hakulinen J, Sankkila L, Sugiyama N et al (2008) Secretion of active membrane type 1 matrix metalloproteinase (MMP-14) into extracellular space in microvesicular exosomes. J Cell Biochem 105:1211–1218. https://doi.org/10.1002/jcb.21923
Reynolds JL, Mahajan SD (2020) Transmigration of tetraspanin 2 (Tspan2) siRNA via microglia derived exosomes across the blood brain barrier modifies the production of immune mediators by microglia cells. J Neuroimmune Pharmacol 15:554–563. https://doi.org/10.1007/s11481-019-09895-6
Ebrahimkhani S, Vafaee F, Young PE et al (2017) Exosomal microRNA signatures in multiple sclerosis reflect disease status. Sci Rep 7:14293. https://doi.org/10.1038/s41598-017-14301-3
Tarhriz V, Eyvazi S, Musavi M et al (2019) Transient induction of Cdk9 in the early stage of differentiation is critical for myogenesis. J Cell Biochem 120. https://doi.org/10.1002/jcb.29204
Zhao Z, Zlokovic BV (2017) Remote control of BBB: a tale of exosomes and microRNA. Cell Res 27:849–850. https://doi.org/10.1038/cr.2017.71
Okoye IS, Coomes SM, Pelly VS et al (2014) MicroRNA-containing T-regulatory-cell-derived exosomes suppress pathogenic T helper 1 cells. Immunity 41:89–103. https://doi.org/10.1016/j.immuni.2014.05.019
Verderio C, Muzio L, Turola E et al (2012) Myeloid microvesicles are a marker and therapeutic target for neuroinflammation. Ann Neurol 72:610–624. https://doi.org/10.1002/ana.23627
Kimura K, Hohjoh H, Fukuoka M et al (2018) Circulating exosomes suppress the induction of regulatory T cells via let-7i in multiple sclerosis. Nat Commun 9:17. https://doi.org/10.1038/s41467-017-02406-2
Lehmann SM, Krüger C, Park B et al (2012) An unconventional role for miRNA: Let-7 activates Toll-like receptor 7 and causes neurodegeneration. Nat Neurosci 15:827–835. https://doi.org/10.1038/nn.3113
Zhou W, Fong MY, Min Y et al (2014) Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell 25:501–515. https://doi.org/10.1016/j.ccr.2014.03.007
Tominaga N, Kosaka N, Ono M et al (2015) Brain metastatic cancer cells release microRNA-181c-containing extracellular vesicles capable of destructing blood-brain barrier. Nat Commun 6:6716. https://doi.org/10.1038/ncomms7716
Pusic AD, Kraig RP (2014) Youth and environmental enrichment generate serum exosomes containing miR-219 that promote CNS myelination. Glia 62:284–299. https://doi.org/10.1002/glia.22606
Pusic AD, Pusic KM, Kraig RP (2014) What are exosomes and how can they be used in multiple sclerosis therapy? Expert Rev Neurother 14:353–355. https://doi.org/10.1586/14737175.2014.890893
Kanninen KM, Bister N, Koistinaho J, Malm T (2016) Exosomes as new diagnostic tools in CNS diseases. Biochim Biophys Acta Mol Basis Dis 1862:403–410. https://doi.org/10.1016/j.bbadis.2015.09.020
Azimi M, Ghabaee M, Moghadasi AN, Izad M (2019) Altered expression of miR-326 in T cell-derived exosomes of patients with relapsing-remitting multiple sclerosis. Iran J Allergy Asthma Immunol 18:108–113. https://doi.org/10.18502/ijaai.v18i1.636
Junker A, Krumbholz M, Eisele S et al (2009) MicroRNA profiling of multiple sclerosis lesions identifies modulators of the regulatory protein CD47. Brain 132:3342–3352. https://doi.org/10.1093/brain/awp300
Ksiazek-Winiarek DJ, Kacperska MJ, Glabinski A (2013) MicroRNAs as novel regulators of neuroinflammation. Mediators Inflamm 2013:1–11. https://doi.org/10.1155/2013/172351
Alexander M, Hu R, Runtsch MC et al (2015) Exosome-delivered microRNAs modulate the inflammatory response to endotoxin. Nat Commun 6:7321. https://doi.org/10.1038/ncomms8321
Nasi M, Bianchini E, de Biasi S et al (2020) Increased plasma levels of mitochondrial DNA and pro-inflammatory cytokines in patients with progressive multiple sclerosis. J Neuroimmunol 338:577107. https://doi.org/10.1016/j.jneuroim.2019.577107
Zhang H, Wu J, Shen FF et al (2020) Activated Schwann cells and increased inflammatory cytokines IL-1β, IL-6, and TNF-α in patients’ sural nerve are lack of tight relationship with specific sensory disturbances in Parkinson’s disease. CNS Neurosci Ther 26:518–526. https://doi.org/10.1111/cns.13282
Chen AQ, Fang Z, Chen XL et al (2019) Microglia-derived TNF-α mediates endothelial necroptosis aggravating blood brain–barrier disruption after ischemic stroke. Cell Death Dis 10:487. https://doi.org/10.1038/s41419-019-1716-9
Dezfulian M (2018) A new Alzheimer’s disease cell model using B cells to induce beta amyloid plaque formation and increase TNF alpha expression. Int Immunopharmacol 59:106–112. https://doi.org/10.1016/j.intimp.2018.04.012
Fujisawa S, Konnai S, Okagawa T et al (2019) Effects of bovine tumor necrosis factor alpha decoy receptors on cell death and inflammatory cytokine kinetics: potential for bovine inflammation therapy. BMC Vet Res 15:68. https://doi.org/10.1186/s12917-019-1813-0
Williams SK, Fairless R, Maier O et al (2018) Anti-TNFR1 targeting in humanized mice ameliorates disease in a model of multiple sclerosis. Sci Rep 8:13628. https://doi.org/10.1038/s41598-018-31957-7
Ribeiro CM, Oliveira SR, Alfieri DF et al (2019) Tumor necrosis factor alpha (TNF-α) and its soluble receptors are associated with disability, disability progression and clinical forms of multiple sclerosis. Inflamm Res 68:1049–1059. https://doi.org/10.1007/s00011-019-01286-0
Dozio V, Sanchez JC (2017) Characterisation of extracellular vesicle-subsets derived from brain endothelial cells and analysis of their protein cargo modulation after TNF exposure. J Extracell Vesicles 6:1302705. https://doi.org/10.1080/20013078.2017.1302705
Chen Y, Sun H, Bai Y, Zhi F (2019) Gut dysbiosis-derived exosomes trigger hepatic steatosis by transiting HMGB1 from intestinal to liver in mice. Biochem Biophys Res Commun 509:767–772. https://doi.org/10.1016/j.bbrc.2018.12.180
Zhen C, Wang Y, Li D et al (2019) Relationship of High-mobility group box 1 levels and multiple sclerosis: a systematic review and meta-analysis. Mult Scler Relat Disord 31:87–92. https://doi.org/10.1016/j.msard.2019.03.030
Doğan HO, Yildiz ÖK (2019) Serum NADPH oxidase concentrations and the associations with iron metabolism in relapsing remitting multiple sclerosis. J Trace Elem Med Biol 55:39–43. https://doi.org/10.1016/j.jtemb.2019.05.011
Becker KA, Halmer R, Davies L et al (2018) Blockade of experimental multiple sclerosis by inhibition of the acid sphingomyelinase/ceramide system. Neurosignals 25:88–97. https://doi.org/10.1159/000484621
van Doorn R, Nijland PG, Dekker N et al (2012) Fingolimod attenuates ceramide-induced blood-brain barrier dysfunction in multiple sclerosis by targeting reactive astrocytes. Acta Neuropathol 124:397–410. https://doi.org/10.1007/s00401-012-1014-4
Li Z, Liu F, He X et al (2019) Exosomes derived from mesenchymal stem cells attenuate inflammation and demyelination of the central nervous system in EAE rats by regulating the polarization of microglia. Int Immunopharmacol 67:268–280. https://doi.org/10.1016/j.intimp.2018.12.001
De Vito F, Balletta S, Caioli S et al (2023) MiR-142–3p is a critical modulator of TNF-mediated Neuronal Toxicity in Multiple Sclerosis. Curr Neuropharmacol 21. https://doi.org/10.2174/1570159x21666230404103914
Gao G, Zhao S, Xia X et al (2019) Glutaminase C regulates microglial activation and pro-inflammatory exosome release: relevance to the pathogenesis of Alzheimer’s disease. Front Cell Neurosci 13. https://doi.org/10.3389/fncel.2019.00264
Chang C, Lang H, Geng N et al (2013) Exosomes of BV-2 cells induced by alpha-synuclein: important mediator of neurodegeneration in PD. Neurosci Lett 548:190–195. https://doi.org/10.1016/j.neulet.2013.06.009
Goetzl EJ, Schwartz JB, Abner EL et al (2018) High complement levels in astrocyte-derived exosomes of Alzheimer disease. Ann Neurol 83:544–552. https://doi.org/10.1002/ana.25172
Bialas AR, Presumey J, Das A et al (2017) Microglia-dependent synapse loss in type i interferon-mediated lupus. Nature 546:539–543. https://doi.org/10.1038/nature22821
Paolicelli RC, Jawaid A, Henstridge CM et al (2017) TDP-43 depletion in microglia promotes amyloid clearance but also induces synapse loss. Neuron 95:297-308.e6. https://doi.org/10.1016/j.neuron.2017.05.037
Heman-Ackah SM, Hallegger M, Rao MS, Wood MJA (2013) RISC in PD: the impact of microRNAs in Parkinson’s disease cellular and molecular pathogenesis. Front Mol Neurosci 6. https://doi.org/10.3389/fnmol.2013.00040
Podbielska M, Szulc ZM, Kurowska E et al (2016) Cytokine-induced release of ceramide-enriched exosomes as a mediator of cell death signaling in an oligodendroglioma cell line. J Lipid Res 57:2028–2039. https://doi.org/10.1194/jlr.M070664
Batlle E, Massagué J (2019) Transforming growth factor-β signaling in immunity and cancer. Immunity 50:924–940. https://doi.org/10.1016/j.immuni.2019.03.024
Stewart AG, Thomas B, Koff J (2018) TGF-β: master regulator of inflammation and fibrosis. Respirology 23:1096–1097. https://doi.org/10.1111/resp.13415
Derynck R, Budi EH (2019) Specificity, versatility, and control of TGF-b family signaling. Sci Signal 12:eaav5183. https://doi.org/10.1126/scisignal.aav5183
Zinski J, Tajer B, Mullins MC (2018) TGF-β family signaling in early vertebrate development. Cold Spring Harb Perspect Biol 10:a033274. https://doi.org/10.1101/cshperspect.a033274
Lee PW, Severin ME, Lovett-Racke AE (2017) TGF-β regulation of encephalitogenic and regulatory T cells in multiple sclerosis. Eur J Immunol 47:446–453. https://doi.org/10.1002/eji.201646716
Zhang YE (2018) Mechanistic insight into contextual TGF-β signaling. Curr Opin Cell Biol 51:1–7. https://doi.org/10.1016/j.ceb.2017.10.001
Yu L, Yang F, Jiang L et al (2013) Exosomes with membrane-associated TGF-β1 from gene-modified dendritic cells inhibit murine EAE independently of MHC restriction. Eur J Immunol 43:2461–2472. https://doi.org/10.1002/eji.201243295
Wong P, Iwasaki M, Somervaille TCP et al (2010) The miR-17-92 microRNA polycistron regulates MLL leukemia stem cell potential by modulating p21 expression. Cancer Res 70:3833–3842. https://doi.org/10.1158/0008-5472.CAN-09-3268
Wong P, Iwasaki M, Somervaille TCP et al (2010) The miR-106b-25 polycistron, activated by genomic amplification, functions as an oncogene by suppressing p21 and Bim. Cancer Res 70:1689–1700. https://doi.org/10.1053/j.gastro.2009.02.002
Wang L, Wang C, Jia X, Yu J (2018) Circulating exosomal miR-17 inhibits the induction of regulatory T cells via suppressing TGFBR II expression in rheumatoid arthritis. Cell Physiol Biochem 50:1754–1763. https://doi.org/10.1159/000494793
Zhang H-G, Liu C, Su K et al (2006) A membrane form of TNF-α presented by exosomes delays T cell activation-induced cell death. J Immunol 176:7385–7393. https://doi.org/10.4049/jimmunol.176.12.7385
Madge LA, Pober JS (2001) TNF signaling in vascular endothelial cells. Exp Mol Pathol 70:317–325. https://doi.org/10.1006/exmp.2001.2368
He F, Peng J, Deng XL et al (2011) RhoA and NF-κB are involved in lipopolysaccharide-induced brain microvascular cell line hyperpermeability. Neuroscience 188:35–47. https://doi.org/10.1016/j.neuroscience.2011.04.025
Wu D, Cerutti C, Lopez-Ramirez MA et al (2015) Brain endothelial miR-146a negatively modulates T-cell adhesion through repressing multiple targets to inhibit NF-κB activation. J Cereb Blood Flow Metab 35:412–423. https://doi.org/10.1038/jcbfm.2014.207
Fenoglio C, Oldoni E, Serpente M et al (2018) LncRNAs expression profile in peripheral blood mononuclear cells from multiple sclerosis patients. J Neuroimmunol 324:129–135. https://doi.org/10.1016/j.jneuroim.2018.08.008
Pahlevan Kakhki M, Nikravesh A, Shirvani Farsani Z et al (2018) HOTAIR but not ANRIL long non-coding RNA contributes to the pathogenesis of multiple sclerosis. Immunology 153:479–487. https://doi.org/10.1111/imm.12850
Oldoni E (2018) The role of cellular and exosomal neural-derived long non coding RNA (LNCRNA) in multiple sclerosis: potential biomarkers of disease susceptibility and progression [PhD thesis]
Duan C, Liu Y, Li Y et al (2018) Sulfasalazine alters microglia phenotype by competing endogenous RNA effect of miR-136-5p and long non-coding RNA HOTAIR in cuprizone-induced demyelination. Biochem Pharmacol 155:110–123. https://doi.org/10.1016/j.bcp.2018.06.028
Song J, Kim D, Han J et al (2015) PBMC and exosome-derived Hotair is a critical regulator and potent marker for rheumatoid arthritis. Clin Exp Med 15:121–126. https://doi.org/10.1007/s10238-013-0271-4
Alexandrov PN, Dua P, Lukiw WJ (2014) Up-regulation of miRNA-146a in progressive, age-related inflammatory neurodegenerative disorders of the human CNS. Front Neurol 5. https://doi.org/10.3389/fneur.2014.00181
Fleshner M, Crane CR (2017) Exosomes, DAMPs and miRNA: features of stress physiology and immune homeostasis. Trends Immunol 38:768–776. https://doi.org/10.1016/j.it.2017.08.002
Stadelmann C, Wegner C, Brück W (2011) Inflammation, demyelination, and degeneration - recent insights from MS pathology. Biochim Biophys Acta Mol Basis Dis 1812:275–282. https://doi.org/10.1016/j.bbadis.2010.07.007
Fancy SPJ, Baranzini SE, Zhao C et al (2009) Dysregulation of the Wnt pathway inhibits timely myelination and remyelination in the mammalian CNS. Genes Dev 23:1571–1585. https://doi.org/10.1101/gad.1806309
Cao P, Feng Y, Deng M et al (2019) MiR-15b is a key regulator of proliferation and apoptosis of chondrocytes from patients with condylar hyperplasia by targeting IGF1, IGF1R and BCL2. Osteoarthr Cartil 27:336–346. https://doi.org/10.1016/j.joca.2018.09.010
Mason JL, Xuan S, Dragatsis I et al (2003) Insulin-like growth factor (IGF) signaling through type 1 IGF receptor plays an important role in remyelination. J Neurosci 23:7710–7718. https://doi.org/10.1523/jneurosci.23-20-07710.2003
Cai H, Chen Y, Yang X et al (2017) Let7b modulates the Wnt/β-catenin pathway in liver cancer cells via downregulated Frizzled4. Tumor Biol 39:1–7. https://doi.org/10.1177/1010428317716076
Jin B, Wang W, Meng XX et al (2016) Let-7 inhibits self-renewal of hepatocellular cancer stem-like cells through regulating the epithelial-mesenchymal transition and the Wnt signaling pathway. BMC Cancer 16:863. https://doi.org/10.1186/s12885-016-2904-y
Wu D, Huang CK, Jiang H (2000) Roles of phospholipid signaling in chemoattractant-induced responses. J Cell Sci 113:2935–2940. https://doi.org/10.1242/jcs.113.17.2935
Salama A, Fichou N, Allard M et al (2014) MicroRNA-29b modulates innate and antigen-specific immune responses in mouse models of autoimmunity. PLoS ONE 9:e106153. https://doi.org/10.1371/journal.pone.0106153
Kim D, Kim TH, Wu G et al (2016) Extracellular release of CD11b by TLR9 stimulation in macrophages. PLoS ONE 11:e0150677. https://doi.org/10.1371/journal.pone.0150677
Liu HY, Huang CM, Hung YF, Hsueh YP (2015) The microRNAs Let7c and miR21 are recognized by neuronal Toll-like receptor 7 to restrict dendritic growth of neurons. Exp Neurol 269:202–212. https://doi.org/10.1016/j.expneurol.2015.04.011
Zhou Y, Wang X, Sun L et al (2016) Toll-like receptor 3-activated macrophages confer anti-HCV activity to hepatocytes through exosomes. FASEB J 30:4132–4140. https://doi.org/10.1096/fj.201600696R
Einarson TR, Bereza BG, Machado M (2017) Comparative effectiveness of interferons in relapsing–remitting multiple sclerosis: a meta-analysis of real-world studies. Curr Med Res Opin 33:579–593. https://doi.org/10.1080/03007995.2016.1276895
Pusic AD, Pusic KM, Clayton BLL, Kraig RP (2014) IFNγ-stimulated dendritic cell exosomes as a potential therapeutic for remyelination. J Neuroimmunol 266:12–23. https://doi.org/10.1016/j.jneuroim.2013.10.014
Lau P, Verrier JD, Nielsen JA et al (2008) Identification of dynamically regulated microRNA and mRNA networks in developing oligodendrocytes. J Neurosci 28:11720–11730. https://doi.org/10.1523/JNEUROSCI.1932-08.2008
Lin ST, Fu YH (2009) miR-23 regulation of lamin B1 is crucial for oligodendrocyte development and myelination. DMM Dis Model Mech 2:178–188. https://doi.org/10.1242/dmm.001065
Budde H, Schmitt S, Fitzner D et al (2010) Control of oligodendroglial cell number by the miR-17-92 cluster. Development 137:2127–2132. https://doi.org/10.1242/dev.050633
Xie W, Li M, Xu N et al (2013) miR-181a regulates inflammation responses in monocytes and macrophages. PLoS ONE 8:e58639. https://doi.org/10.1371/journal.pone.0058639
Hutchison ER, Kawamoto EM, Taub DD et al (2013) Evidence for miR-181 involvement in neuroinflammatory responses of astrocytes. Glia 61:1018–1028. https://doi.org/10.1002/glia.22483
Ponomarev ED, Veremeyko T, Weiner HL (2013) MicroRNAs are universal regulators of differentiation, activation, and polarization of microglia and macrophages in normal and diseased CNS. Glia 61:91–103. https://doi.org/10.1002/glia.22363
Pusic KM, Pusic AD, Kraig RP (2016) Environmental enrichment stimulates immune cell secretion of exosomes that promote CNS myelination and may regulate inflammation. Cell Mol Neurobiol 36:313–325. https://doi.org/10.1007/s10571-015-0269-4
Sarchielli P, di Filippo M, Ercolani MV et al (2008) Fibroblast growth factor-2 levels are elevated in the cerebrospinal fluid of multiple sclerosis patients. Neurosci Lett 435:223–228. https://doi.org/10.1016/j.neulet.2008.02.040
Paul A, Comabella M, Gandhi R (2019) Biomarkers in multiple sclerosis. Cold Spring Harb Perspect Med 9:51–58. https://doi.org/10.1101/cshperspect.a029058
Maciotta S, Meregalli M, Torrente Y (2013) The involvement of microRNAs in neurodegenerative diseases. Front Cell Neurosci 7. https://doi.org/10.3389/fncel.2013.00265
de Felice B, Mondola P, Sasso A et al (2014) Small non-coding RNA signature in multiple sclerosis patients after treatment with interferon-β. BMC Med Genomics 7:26. https://doi.org/10.1186/1755-8794-7-26
Potenza N, Mosca N, Mondola P et al (2018) Human miR-26a-5p regulates the glutamate transporter SLC1A1 (EAAT3) expression. Relevance in multiple sclerosis. Biochim Biophys Acta Mol Basis Dis 1864:317–323. https://doi.org/10.1016/j.bbadis.2017.09.024
Riazifar M, Mohammadi MR, Pone EJ et al (2019) Stem cell-derived exosomes as nanotherapeutics for autoimmune and neurodegenerative disorders. ACS Nano 13:6670–6688. https://doi.org/10.1021/acsnano.9b01004
Ziemssen T, Akgün K, Brück W (2019) Molecular biomarkers in multiple sclerosis. J Neuroinflammation 16:S10-S009. https://doi.org/10.1186/s12974-019-1674-2
Niwald M, Migdalska-Sęk M, Brzeziańska-Lasota E, Miller E (2017) Evaluation of selected microRNAs expression in remission phase of multiple sclerosis and their potential link to cognition, depression, and disability. J Mol Neurosci 63:275–282. https://doi.org/10.1007/s12031-017-0977-y
Mohammadinasr M, Montazersaheb S, Molavi O et al (2023) Multiplex analysis of cerebrospinal fluid and serum exosomes microRNAs of untreated relapsing remitting multiple sclerosis (RRMS) and proposing noninvasive diagnostic biomarkers. Neuromolecular Med. https://doi.org/10.1007/s12017-023-08744-3
Cao Q, Guo Z, Yan Y et al (2019) Exosomal long noncoding RNAs in aging and age-related diseases. IUBMB Life 71:1846–1856. https://doi.org/10.1002/iub.2141
Selmaj I, Cichalewska M, Namiecinska M et al (2017) Global exosome transcriptome profiling reveals biomarkers for multiple sclerosis. Ann Neurol 81:703–717. https://doi.org/10.1002/ana.24931
Giovannelli I, Martelli F, Repice A et al (2015) Detection of JCPyV microRNA in blood and urine samples of multiple sclerosis patients under natalizumab therapy. J Neurovirol 21:666–670. https://doi.org/10.1007/s13365-015-0325-3
Acknowledgements
This is a report of the database from a Ph.D. thesis registered in Tabriz University of Medical Sciences with the Number 62877.
Funding
This study was supported by Molecular Medicine Research Center, Tabriz University of Medical Sciences (number 62877), Iran.
Author information
Authors and Affiliations
Contributions
The core idea of this study came from M.M. and M.S.H. M.M. and S.M. collected the papers. All authors were involved in analyzing the collected papers. M.M., S.M., and M.S.H. wrote the manuscript in collaboration with H.A., V.H., and O.M. Final editing was done by M.M., S.M., H.A., V.H., O.M., and M.S.H. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Approval was granted by the Ethics Committee of Tabriz University of Medical Sciences (IR.TBZMED.REC.1397.349).
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mohammadinasr, M., Montazersaheb, S., Ayromlou, H. et al. Exosome Content–Mediated Signaling Pathways in Multiple Sclerosis. Mol Neurobiol (2024). https://doi.org/10.1007/s12035-023-03862-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12035-023-03862-2