Abstract
Viral infections of the central nervous system (CNS) cause variable outcomes from acute to severe neurological sequelae with increased morbidity and mortality. Viral neuroinvasion directly or indirectly induces encephalitis via dysregulation of the immune response and contributes to the alteration of neuronal function and the degeneration of neuronal cells. This review provides an overview of the cellular and molecular mechanisms of virus-induced neurodegeneration. Neurotropic viral infections influence many aspects of neuronal dysfunction, including promoting chronic inflammation, inducing cellular oxidative stress, impairing mitophagy, encountering mitochondrial dynamics, enhancing metabolic rewiring, altering neurotransmitter systems, and inducing misfolded and aggregated pathological proteins associated with neurodegenerative diseases. These pathogenetic mechanisms create a multidimensional injury of the brain that leads to specific neuronal and brain dysfunction. The understanding of the molecular mechanisms underlying the neurophathogenesis associated with neurodegeneration of viral infection may emphasize the strategies for prevention, protection, and treatment of virus infection of the CNS.
Similar content being viewed by others
Background
Neurodegeneration is the progressive atrophy and loss of function of neurons, glial cells, and the neural networks in the brain and spinal cord. This degeneration and death of neurons often leads to several neurodegenerative diseases that cause complex and serious medical conditions that worsen over time with motor, cognitive, and autonomic dysfunction, such as Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), amyotrophic lateral sclerosis (ALS), and prion diseases [1]. Different neuropathologic mechanistic processes cause neuronal death in neurodegenerative diseases including the oxidative stress due to the overproduction of free radicals with the decline of cellular antioxidant defense systems. Excessive free radical formation induces mitochondrial dysfunction, mitochondrial dynamic imbalance, and impaired bioenergetics which contribute to the redox imbalance that can cause malfunctioning of the endoplasmic reticulum (ER), resulting in the abnormal protein accumulation, calcium accumulation, and intrinsic cell death pathway activation [2]. Severe or prolonged stress activates cell death pathways, including autophagy and apoptosis [3]. Neuronal loss in neurodegenerative diseases is often associated with the deposition of extra- and intracellular misfolded proteins that are toxic to neurons, impair mitochondrial redox activity, and increases the generation of oxidative stress that activates apoptosis signaling to trigger cell death [4]. The activation of an immune response by microglia and astrocytes which occurs in response to the cytotoxic consequences of the aggregation of misfolded proteins induces the production of several inflammatory factors that promote chronic neuroinflammation associated with the progression of neuronal loss in the CNS [5]. These interrelated mechanisms are involved in the death and dysfunction of neurons in neurodegenerative diseases.
Neurotropic viruses are common causes of CNS infections, both acute and chronic viral neurologic syndromes, including meningitis, encephalitis, encephalomyelitis, and myelitis [6]. Viruses can enter the CNS via two major routes: blood circulation (viremia) and crossing the blood–brain barrier (BBB) or entering via peripheral nerve endings. Viral entry into the brain through the BBB can occur through three different mechanisms: the transcellular pathway (virus passage through infected endothelial cells), the “Trojan Horse” pathway (virus transport using infected immune cells), or the paracellular pathway (virus entry through disruption of junction proteins, the actin cytoskeleton, or the basal lamina) [7, 8]. The invasion of neurotropic viruses into the CNS is associated with neurodegeneration through a variety of mechanisms. Viruses primarily infect neurons through interaction with host attachment factors and receptors, followed by either direct fusion to the plasma membrane (enveloped viruses) or endocytosis (non-enveloped viruses) and subsequent delivery of the viral genome (DNA or RNA) to the cytosol or the nucleus of the infected cell [9]. Upon entry, the virus replicates within neuronal cells, and the accumulation of viral antigens induces an increase in oxidative stress which leads to the activation of both innate and adaptive immune responses. Viral components are recognized by host pattern recognition receptors (PRRs) such as Toll-like receptors (TLRs), C-type lectin receptors (CLRs), NOD-like receptors (NLRs), Retinoic acid-inducible gene-I (RIG-I)-like receptors (RLRs), and cytosolic DNA sensors (CDS) which are widely expressed in the CNS cells including microglia, neurons, astrocytes, oligodendrocytes, epithelial cells, and innate immune cells [7]. Activation of PRR signaling triggers the production of inflammatory mediators in order to clear viral invasion. However, chronic activation of these receptors can cause inflammatory damage [10]. Neurotropic virus infection induces neuronal damage through direct killing, cell lysis, increased free radical release, perturbation of the cellular stress response, cellular activation of neuroinflammation, and induction of apoptotic signaling leading to neuronal cell death [11, 12]. In addition, viruses can infect neuroglial cells, which results in the activation of astrocytes and microglia, leading to the production of numerous proinflammatory cytokines. The overproduction of proinflammatory cytokines induces an increase in the permeability of the BBB, which allows the virus to easily enter the CNS. Moreover, the increase in proinflammatory cytokines induces neuroinflammation, which leads to pathological changes such as cellular infiltration, perivascular cuffing meningeal disruption, neuronal shrinkage, and plaque formation in brain tissues. Viral infections disrupt the cellular function of neurons, such as metabolic pathways and neurotransmitter synthesis, which result in neuronal and brain dysfunctions. Viral infections implicate pathogenic etiology in neurodegenerative diseases. In this review, we provide and summarize the molecular mechanisms of neuropathogenesis associated with neurotropic virus-induced neurodegeneration.
Viral Infection Induces Oxidative Stress
Infection with neurotropic viruses promotes oxidative stress, which is associated with excessive production of reactive oxygen species (ROS) and insufficient cellular antioxidant defenses. The disturbance in the oxidant–antioxidant balance leads to potential cellular damage in the host cell. ROS can damage cell components such as nucleic acids, lipids, and proteins and subsequently disturb their functions, contributing to neurodegeneration conditions in the CNS. Mitochondria are the major source of intracellular ROS and antioxidant enzymes, which maintain redox balance. Neurotropic viruses causing both acute and slow infection can induce the generation of ROS, which can initiate the lipid peroxidation process and play a critical role in cellular death (Fig. 1).
Neurotropic viral infection induces neurodegeneration through a variety of cellular mechanisms. Viral infection affects several host cell response mechanisms to attenuate neuronal functions, including (i) increasing reactive oxygen species (ROS) by interfering with the electron transport system and disrupting the production of antioxidants, (ii) promoting neuroinflammation by increasing proinflammatory cytokine secretion from infiltrated inflammatory cells and infected neuronal cells such as microglia and astrocytes, which then (iii) activates the apoptosis signaling pathway by inducing both intrinsic and extrinsic signaling pathways leading to neuronal death in the CNS. Abbreviation: Cyt c, cytochrome c; CoQ, coenzyme Q; BCL-2, B-cell lymphoma 2; BAX, BCL-2 associated X; FAD, flavin adenine dinucleotide; FADH, flavin adenine dinucleotide hydride; NADH, reduced nicotinamide adenine dinucleotide; NAD+, nicotinamide adenine dinucleotide; TNF-α, tumor necrosis factor alpha; TRADD, TNF receptor-associated death domain; CAT, catalase; SOD, superoxide dismutase; GPx, glutathione peroxidase; GR, glutathione reductase; TRX, thioredoxin; TLRs, toll-like receptors; TCA, tricarboxylic acid
Japanese encephalitis virus (JEV; family Flaviviridae) infection can cause Japanese encephalitis in humans with a high fatality rate in severe cases, and ~30–50% of survivors experience serious permanent neurologic sequelae or psychiatric sequelae [13, 14]. JEV infection increases the level of ROS production, which increases neuronal cell death triggered by both mature viruses and replication-incompetent virions [15]. ROS overproduction together with a decrease in membrane fluidity in JEV-infected neuronal cells causes severe cytopathic effects and subsequently contributes to neuronal cell death [16]. JEV infection increases the levels of superoxide anions (O2.-), nitric oxide (NO), and peroxynitrite (OONO-) in neurons and glial cells [17,18,19]. Excessive O2.- production during viral infection was also observed in neuronal cells infected with other members of Flaviviridae family, including West Nile virus (WNV) [20] and dengue virus type 2 (DENV-2) [21], leading to host cell apoptosis.
Venezuelan equine encephalitis virus (VEEV; family Togaviridae) causes severe zoonotic disease in humans, and approximately 4–14% of cases develop serious neurological complications and 1% develop lethal encephalitis [22,23,24]. VEEV infection significantly increases in ROS levels in astocytoma U87MG cells [25]. An increase in NO formation together with the activation of inducible nitric oxide synthase (iNOS) was observed in the brains of mice infected with VEEV [26]. The overproduction and activation of malondialdehyde (MDA), a lipid peroxidation marker, was detected in the brains of JEV-infected animals [27]. Lipid peroxidation induced by VEEV has been shown to increase the concentration of thiobarbituric acid reactive substances (TBARS), a byproduct of lipid peroxidation, in the mouse brain [26].
Rabies virus (RABV; family Rhabdoviridae) is a deadly virus that infects the CNS and causes encephalitis, ultimately resulting in death in mammals [28]. RABV infection is shown to increase the ROS production in mouse neuroblastoma cells [29]. The viral component of RABV plays a critical role in the induction of oxidative stress [30]. Immunostaining for 4-hydroxy-2-nonenal (4-HNE) indicated evidence of lipid peroxidation associated with oxidative stress that causes axonal injury, as shown by axonal swelling and reduced axonal growth in the dorsal root ganglion of RABV-infected mice [31]. Another member of the Rhabdoviridae family, Chandipura virus (CHPV), an enveloped RNA virus that causes acute encephalitis mainly in children, has been reported to induce neuronal apoptosis by stimulating oxidative stress. CHPV infection increases the intracellular Ca2+ secretion, which further increases ROS, superoxide production levels, and mitochondrial dysfunction within CHPV-infected cells and causes neuronal death in vitro and in vivo [32,33,34].
Enterovirus 71 (EV71; family Picornaviridae) is a major causative agent of hand, foot, and mouth disease (HFMD) with fatal neurological complications in young children. EV71 infection can lead to increased ROS generation and activation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, which in turn enhances EV71 infection in neural cells [35,36,37].
Oxidative stress is also seen in viruses causing latent or slow infections, such as herpes simplex virus (HSV; family Herpesviridae) and human immunodeficiency virus (HIV; family Retroviridae). Herpes simplex encephalitis is caused by herpes simplex virus type 1 (HSV-1) and is a common cause of sporadic focal encephalitis worldwide [38]. Intracellular ROS generation in response to HSV-1 infection was observed in microglia [39] and neural cells [40]. HSV-1 infection causes oxidative stress and induces the release of bioactive lipid peroxidation byproducts, MDA/hydroxyalkenals (HAEs), in cultured mouse neural cells, which is necessary for virus replication [40]. HIV causes immunodeficiency, which leads to acquired immunodeficiency syndrome (AIDS). HIV infects the CNS and enhances neurotoxicity, which directly harms the brain and manifests as HIV-associated neurocognitive disorders (HANDs) [41,42,43]. Several component proteins of human immunodeficiency virus type-1 (HIV-1) enhance ROS production in neuronal cells, including neurons, microglial cells, and astrocytes, by different mechanisms [44,45,46]. The HIV-1 transactivator of transcription (Tat) protein induces the production of ROS and significantly induces DNA breakage [47]. Increased levels of nitroxidative stress marker proteins such as NADPH oxidase, cytochrome P450-2E1 (CYP2E1), and iNOS are observed in HIV-1 transgenic rat brains [48].
ZIKV targeting of neuronal cells causes neurological complications such as congenital microcephaly, Guillain–Barré syndrome, transverse myelitis, and meningoencephalitis [49, 50]. ZIKV impairs mitochondrial structure and function, as shown by the decrease in oxygen flux coupled with adenosine triphosphate synthesis [51]. ZIKV infection leads to the increased production of ROS, which is associated with DNA breakage in neural cells in vivo and in vitro [51, 52]. ZIKV infection resulted in a significant increase in lipid peroxidation, as observed by the high levels of MDA and carbonyl protein in human glioblastoma-infected cells [52].
The increase in free radicals and lipid peroxidation induced by neurotrophic viruses demonstrates that oxidative stress contributes as a key factor in the pathogenesis of neurodegeneration in viral infection of the CNS.
Virus Infection Disturbs the Production of Antioxidants
The elevation of cellular oxidative stress due to increases in free radicals and lipid peroxidation was associated with an opposite decrease in antioxidant activities. Viral infections stimulate ROS production and inhibit antioxidant enzyme levels [53, 54] (Fig. 1). The antioxidant defense mechanism is impaired by a decline in intracellular antioxidant levels, such as superoxide dismutase 1 (SOD-1), thioredoxin 1 (TRX-1), and glutathione (GSH), and a reduction in catalase (CAT) and glutathione peroxidase (GPx) activities in neuronal cells and several brain regions during JEV infection [16, 27]. EV71 diminished the ratio of GSH to its disulfide form, glutathione disulfide (GSSG), an indicator of oxidative stress [55]. ZIKV-induced oxidative stress is correlated with decreased levels of SOD and CAT activities in U87-MG cells and the brains of C57BL/6 mice [52]. Infection with HSV-1 has been reported to induce the depletion of GSH [56]. Exposure of rat brain endothelial cells to HIV-1 envelope glycoprotein GP120 (gp120) and Tat significantly decreases the levels of intracellular GSH, GPx, and glutathione reductase (GR) and the ratio of GSH/GSSG [57]. The decrease in antioxidant enzyme activities after ZIKV infection was found to be associated with the negative regulation of nuclear factor erythroid 2-related factor 2 (NRF2)/antioxidant response element (ARE) signaling [52]. This evidence suggests that depletion of the antioxidant enzymatic system occurs during viral infection.
Virus Infection Induces Mitochondrial and Endoplasmic Reticulum Stresses
Mitochondrial oxidative stress is involved in pathological statuses and is the major source of excessive amounts of ROS in infected cells. ROS accumulation in cells is a direct reflection of disruption in mitochondrial electron transport chain (ETC) function and redox imbalance in the ER lumen, leading to the accumulation of unfolded proteins and in turn increasing oxidative stress, which results in increased cellular damage and apoptosis. An increase in ETC-related protein activity was observed after viral infection (Fig. 1). RABV infection significantly alters a variety of mitochondrial parameters, such as increases in maximal uncoupled respiration and complex IV respiration and mitochondrial complex I and complex IV activities in neurons [58]. The activity of mitochondrial complex I, a site of ROS production, was correlated with the high level of ROS in mouse neuroblastoma cells [29]. The increase in the generation of ROS and oxidative stress caused by the specific 139–172 region of the RABV phosphoprotein that interacts with complex I in mitochondria causes mitochondrial dysfunction [29]. RABV infection induces mitochondrial ETC dysfunction, resulting in oxidative stress and degenerative changes in neuronal processes (involving both dendrites and axons) in infected mice [31, 59, 60]. Experiments using inhibitors of several mitochondrial proteins showed that EV71 mainly induces ROS generation at a site of the mitochondrial ETC distal to mitochondrial complex III [35]. Increased mitochondrial oxidative stress by EV71 infection can cause mitochondrial morphological changes and exhibit functional anomalies, such as a decrease in mitochondrial electrochemical potential and a lower respiratory control ratio of mitochondria in glioblastoma cells [35].
Together with mitochondrial oxidative stress, ER stress usually occurs simultaneously to generate cellular stress in virus-infected cells. The ER is responsible for the correct three-dimensional conformation of protein folding and maturation. The formation of disulfide bonds produces large amounts of ROS and depletes GSH, contributing to the redox imbalance that can cause malfunctioning of the ER, including massive protein production, loss of Ca2+ homeostasis, inhibition of N-linked glycosylation, and accumulation of mutant proteins [61, 62]. Viral replication can cause an increase in viral protein synthesis demand to overcome the ER folding capacity, leading to the massive production of misfolded viral proteins that accumulate in the ER lumen and trigger ER stress [63]. JEV infection induces ER stress as evidence of the detection of the excessive proliferation of ER membranes together with the amount of viral proteins and the induction of unfolded protein response (UPR) signaling in neuronal N18 and NT-2 cells [16]. In human neural stem cells (hNS1 cells), JEV infection promoted the expression of ER stress-related proteins such as glucose-regulated protein 78 kDa (GRP78), heat shock protein (HSP) 60, HSP70, and HSP90 [64]. JEV infection activates several ER stress sensors, including protein kinase RNA-like ER kinase (PERK), activating transcription factor 4 (ATF4), and C/EBP homologous protein (CHOP) under acute or prolonged ER stress. The JEV-induced UPR provokes CHOP/growth arrest and DNA damage-inducible protein (GADD153) and triggers the activation of p38 mitogen-activated protein kinase (MAPK), which enhances JEV-induced apoptosis via the activation of the caspase cascade [16]. JEV nonstructural protein 4B (NS4B) activates the PERK/ATF4/CHOP neuronal apoptosis pathway both in vitro and in vivo [65].
WNV infection activates multiple branches of ER stress-mediated UPR pathways, leading to transcriptional and translational induction of UPR target genes [63]. Activation of ATF6 and PERK pathways was induced during WNV infection in SK-N-MC neuroblastoma cells, resulting in CHOP activation and downstream apoptosis [66]. The transcriptomic analysis shows that the components of the UPR-related genes such as PERK, ATF4, and DDIT3 (encoded CHOP) and early growth response 1 (EGR1) are activated in VEEV-infected human astrocytoma cells [67]. The activation of EGR1 is regulated by extracellular-signal-regulated kinase (ERK) and PERK pathways, which are important in contributing to neural cell death in VEEV infection [68].
ZIKV infection activates ER stress by significantly increasing the expression of ER stress markers in neural cells [69]. ZIKV upregulates UPR-related genes in the cerebral cortex of infected postmortem human fetuses as well as in cultured human neural stem cells and in the mouse embryonic brain [70]. Transfection with EV71 viral envelope protein 1 (VP1) increased translation initiation factor 2α (eIF2α) kinase phosphorylates Serine51 (Ser51) phosphorylation, which represented to ER stress activation in mouse brainstem neurons [71]. In mouse Schwann cells, the overexpression of VP1 also induced ER stress, leading to the upregulation of peripheral myelin protein 22 (PMP22) [72]. These observations indicate the role of ER stress in the pathogenesis induced by viral infection.
Virus Infection Induces Alteration of Autophagy
The cellular stress response induced by virus infection is also related to the induction of autophagy in infected cells [73]. CHPV infection shows an increase in microtubule-associated protein 1 light chain 3 beta (LC3B), an autophagic marker, associated with overproduction of ROS and mitochondria dysfunction in HT-22 mouse hippocampal neuronal cell [32]. Infection with EV71 shows an increase in LC3-II protein as well as the formation of LC3 aggregates, autophagosomes, and amphisomes in several infected brain tissues of mice [74, 75]. Immunofluorescence staining indicated the colocalization of EV71 proteins with LC3 and mannose-6-phosphate receptor (MPR, endosome marker) proteins, which indicates amphisome formation accompanied by autophagic flux in EV71-infected SK-N-SH cells [74]. The EV71 VP1 protein is an important neurovirulence protein that induces autophagy by regulating the mammalian target of rapamycin (mTOR) signaling pathway to promote viral replication [75,76,77]. Phosphorylated mTOR, phosphorylated 70-kDa S6 kinase (p70S6K), and peroxisomal acyl-coenzyme A oxidase 1 (ACOX1) are signaling pathways involved in EV71-induced autophagy and neural cell apoptosis when downregulated [36, 75, 76]. In HIV-1 associated encephalitis, increases in several autophagic markers, such as Beclin-1, autophagy-related gene (Atg)-5, Atg-7, and LC3-II, have been observed in frontal cortex postmortem brains [78]. The SK-N-SH cells treated with gp120 from C-X-C motif chemokine receptor 4 (CXCR4) and C-C motif chemokine receptor 5 (CCR5)-tropic HIV-1 virus exhibit the accumulation of autophagic proteins and autophagosomes [78]. Autophagy in HAND has also been evaluated. HIV-1 proteins such as Nef and Tat alter neural autophagy in different manners related to the autophagosome formation and autophagic flux, which may contribute to HAND [79,80,81]. The JEV-activated autophagy has been explored in both N2a neuroblastoma cells and mouse brains, and it demonstrated an increase in LC3-II protein accumulation and induction of autophagosome formation [82].
Virus Infection Induces Impairment of Mitophagy and Mitochondrial Dynamics
In addition, viruses induced impairment of mitochondrial dynamics and triggered mitophagy, a specific autophagy form for the removal of damaged mitochondria, directly and indirectly, and controlled the mitophagic process via different strategies [83]. The accumulation of ROS by VEEV TC-83 infection alters mitochondrial dynamics by affecting the expression of dynamin-related protein 1 (DRP1), a protein that plays a role in mitochondrial fission, resulting in an increase in mitochondrial fractions in infected astrocytes [25]. PTEN-induced kinase 1 (PINK1) and Parkin, proteins associated with mitophagy, appear to be enriched in mitochondrial fractions, indicating that mitochondrial damage contributes to the apoptosis of infected cells [25]. As shown in human primary neurons, HIV-1 gp120 and Tat proteins induce the mitochondrial fission process via DRP1 as a result of neural mitochondrial fragmentation and then activate mitophagy markers such as LC3B and Beclin-1 and recruit PINK and Parkin sequestosome 1 (SQSTM1) to damaged mitochondria [84,85,86,87]. However, HIV-1 proteins are found to inhibit mitophagic flux in human primary neurons by impairing the delivery of mitochondria to the lysosomal compartment, leading to incomplete neuronal mitophagy, which causes neuronal damage [84]. Dysregulation of autophagy and mitophagy and alteration of mitochondrial dynamics may be important and contribute to the pathogenesis of neurotropic virus infection.
Virus Infection Activates the Apoptosis Signaling Pathway
Oxidative stress elicits the loss of mitochondrial membrane potential and results in the induction of the intrinsic apoptosis pathway [16, 88, 89]. The expression of apoptotic protein markers has been observed in several neurotropic virus-infected cells (Fig. 1). Overexpression of caspase-3 is observed in the brains of pediatric patients with HIV-1 encephalitis and corresponds to increases in DNA fragmentation, a marker of apoptotic cells [90]. In the HIV-1 model, the levels of BCL2 associated X (BAX) and activated caspase-3 were significantly elevated in the hippocampus and were associated with neuronal cell death in HIV-1 transgenic rats [48]. HIV-1 gp120 protein induces apoptosis in neurons and microglial cells in association with the activation of MAPK pathways mediated by ERK and JNK and lowering the expression of B-cell lymphoma 2 (BCL-2) [42, 91, 92]. BCL-2 is a major target of HIV-induced changes that are modulated to different degrees during HIV infection, resulting in either a proapoptotic or an antiapoptotic phenotype [93]. VEEV infection causes neuronal injury ranging from nuclear chromatin condensations to nuclear and cellular fragmentation, indicating apoptotic cell death [94]. The positron emission tomography (PET) with a tracer targeting the caspase-3 substrate revealed an increase in apoptosis and a decrease in BBB integrity in VEEV-infected mouse brains [95]. EV71 markedly reduces BCL-2 expression but induces an increase in the mRNA expression of several apoptosis-promoting factors, such as BAX, CASP7 (caspase-7), CASP3 (caspase-3), and cleaved caspase-3 [96]. EV71 infection triggers the translocation of cytochrome c (Cyt c) from mitochondria to the cytosol, and caspase-9 is activated, leading to neural cell death and indicating that the mitochondria-mediated intrinsic apoptotic pathway is activated by EV71 [97, 98]. EV71 also induces cell cycle arrest of SH-SY5Y cells through stimulation of endogenous microRNA let-7b expression after EV71 infection [96]. The invasion of RABV into the CNS shows morphologic changes of apoptosis and marked increases in BAX in the hippocampus and cerebral cortex [99,100,101,102,103]. The marked apoptotic are present in neurons, glial cells, and perivascular mononuclear cells within the white matter of the cerebellum of dogs with the positive detection of BCL-2 and BAX protein [104]. The morphological changes and apoptosis of spinal neurons and dorsal root spinal ganglion cells are present in the late period of infection [105]. The replication of RABV in mouse neuroblastoma cells increases the level of BAX and caspase activation, which induces the degradation of poly ADP-ribose polymerase (PARP), leading to destruction of the DNA [106]. WNV proteins such as capsid and nonstructural protein 3 (NS3) induce apoptotic features in CNS cells via BAX-dependent apoptosis that triggers mitochondria-outer-membrane-permeabilization [107,108,109,110,111]. WNV replication decreased cell viability and induced upregulation of BAX expression and the release of Cyt c from the mitochondria and formation of apoptosomes, followed by the activation of the effector caspase-3, the initiator caspases-8 and -9 then cleavage of the PARP in which induces neuronal cell death [66, 108, 111,112,113,114]. JEV infection promoted increased the expression of BAX [89, 115], cleaved PARP levels [88], and the activation of caspase-3 activity [116], were observed in JEV-infected cells (Fig. 1). JEV infection also increased Cyt c release and caspase 3 activation in cultured neuronal cells and in infected mouse brain, inducing neuronal death, and increasing the mortality rate of mice [19, 115, 117]. A reduction in BCL-2 expression levels was found in JEV-infected neuronal cells and the mouse brain [115]. Several studies have also reported the overexpression of BCL-2 levels at the early stage of JEV infection, which helps to the induce host cell survival in order to facilitate viral persistence [117,118,119,120,121]. However, the studies showed that the increase in BCL-2 during JEV infection failed to inhibit the infected cells from undergoing apoptosis and failed to block viral replication [117, 119, 121, 122]. Moreover, our recent study with a high multiplicity of infection (MOI) of Beijing-1 infection remarkably induced neuronal cell death via inducing the proteolysis of endogenous p21 BAX to generate more apoptogenic molecules of p18 BAX during the late stage of JEV infection [117]. The accumulation of cleaved p18 BAX might be due to the activation of calpain protease, which increases the intrinsic cytotoxic properties of this proapoptotic molecule and effectively induces Cyt c release from mitochondria, leading to the activation of the caspase cascade, which results in the induction of intrinsic apoptosis [123]. Thus, the intrinsic apoptosis pathway plays an important role in the pathogenesis of neurotropic virus infection.
Virus Infection Induces Neuroinflammation
Neuroinflammation is an important feature of neurodegenerative disorders. Normally, neuroinflammatory processes play a protective role in response to CNS injury by several factors, including viral infections. Neurotropic viral infection of the CNS can cause the activation of microglia and astrocytes, inducing the production of several neuroinflammatory factors that promote chronic inflammation and increasing the progression of CNS diseases by the virus [124].
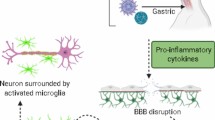
Inflammation is a hallmark of virus encephalitis. During JEV infection, various peripheral immune cell types infiltrate the CNS and affect the integrity of the BBB, which is a major regulator of neuroinflammation and viral propagation into the brain [125, 126]. In addition, peripheral immune cells infiltrating brain-resident cells interact with JEV upon infection of the brain. Microglial cells are the resident macrophages of the CNS, can be productively infected by JEV, and might serve as a reservoir for the virus [127]. The activation of microglia has been proposed to play a major role in neuronal cell death through the release of proinflammatory mediators [125, 128, 129]. The upregulation of TLR3, TLR7, and RIG-I signaling, including ERK, p38MAPK, activator protein 1 (AP-1), and nuclear factor kappa B (NF-κB), are triggered by JEV infection and induces the activation of microglia [116, 129] (Fig. 1). JEV induces encephalopathy by activating microglial cells and astrocytes that can increase the levels of proinflammatory cytokines such as tumor necrosis factor alpha (TNF-α), interleukins (IL-6, IL-8 and IL-10), and RANTES (regulated on activation, normal T-cell expressed and secreted) [130, 131].
Overproduction of chemokines and proinflammatory cytokines are key factors that play an important role in the pathogenesis of flavivirus infections. In the brain, the release of chemokines regulates the migration of leukocytes from the peripheral into the site of infection. Both clinical and experimental models suggest the involvement of neuroinflammation in dengue virus disease [132]. Intracranial injection of DENV is associated with the induction of interferon-beta (IFN-β), interferon-gamma (IFN-γ), TNF-α, chemokine (C-C motif) ligand 2 (CCL-2), CCL-5, C-X-C motif chemokine (CXCL)1, CXCL2, and IFN-stimulated gene (ISG) expression including viperin, Ifi27l2a, IRF7, and CXCL10 [133, 134]. DENV-2 and DENV-3 infections enhance of the infiltration of CD8+ and CD4+ T cells and neutrophils in the brain [132,133,134]. High levels of chemokines and proinflammatory cytokines such as IL-6, IL-1β, IFNγ, and TNF-α were associated with the severity of neuronal damage and the high mortality rate of mice during JEV infection [19, 131, 135,136,137]. TNF-α has a major effect on the induction of neuronal cell death in JEV infection by inducing the extrinsic apoptosis pathway via TNF receptor-associated death domain (TRADD) [89] and triggering inflammatory cells to release other cytokines, resulting in neuronal cell death [19, 131]. Moreover, IL-6, CXCL10, CCL-2, and CCL-5 downregulated the expression of BBB tight junction proteins, causing an increase in BBB permeability [136]. In addition, IL-1β activates the expression of adhesion molecules on epithelial cells, leading to inflammatory cell infiltration and the subsequent generation of numerous proinflammatory mediators, which ultimately cause neuronal cell death and irreversible brain damage [131, 136].
In another encephalitis virus, VEEV, PET tracer was used to target 18-kDa translocator protein (TSPO) to study the accumulation of macrophages and microglia/astrocyte activation; the results demonstrated an increase in neuroinflammation in the cortex, thalamus, striatum, hypothalamus, hippocampus, olfactory bulb, brain stem, and cerebellum of VEEV, and a decrease in BBB integrity was observed in VEEV-infected mouse brains [95]. VEEV-infected microglia produce several proinflammatory cytokines as a result of direct infection, including IFNγ, IL-1α, IL-1β, IL-6, and IL-12 [138].
The secretion of the proinflammatory cytokines, IFNγ, IL-6, and IL-1β in the cerebrospinal fluid (CSF) was increased in the acute stage of CNS involvement from EV71 infection [139, 140]. The histological studies of EV71-infected autopsy tissues showed obvious inflammation in the spinal cord gray matter, brainstem, hypothalamus, and subthalamic and dentate nuclei with perivascular cuffs, variable edema, neuronophagia, and microglial infiltration [141, 142]. EV71 infection increases TNF-α, IL-1β and RANTES production, which triggers bystander damage to neurons involving the tyrosine kinase/MAPKs/NF-κB signaling cascade during EV71 infection [143].
Tick-borne encephalitis virus (TBEV; family Flaviviridae) is an important tick-transmitted virus that causes tick-borne encephalitis (TBE) in Eurasia [144]. Increased levels several cytokines and chemokines have been detected in CSF or serum samples from TBE patients with higher ratios of IL-12:IL-4 and IL-12:IL-10, reflecting the global pro-inflammatory cytokine balance [145,146,147,148]. TBEV disrupts the BBB and infects neurons, astrocytes, and oligodendrocytes, inducing neuroinflammation followed by neuronal death [149,150,151]. TBEV infection upregulates the expression of several pathogen recognition receptors, pro-inflammatory cytokines, and interferon-stimulated genes in neuronal/glial cells [152, 153]. TBEV-infected mice exhibited time-dependent increases in serum and brain tissue concentrations of multiple cytokines/chemokines such as CXCL10/IP-10, CXCL1, G-CSF IL-6, and RANTES [153, 154]. Thus, neurons and astrocytes are potential sources of pro-inflammatory cytokines in TBEV infection (Fig. 1).
The CNS is the major target of ZIKV infection. In addition to the induction of massive neuronal cell damage, ZIKV-mediated neuroinflammation is the key to causing neurological pathology via an excessive inflammatory response, particularly in neonates. The relationship between ZIKV-induced neuroinflammation and postnatal microcephaly has been revealed in animal models and fatal cases of ZIKV-associated microcephaly [155, 156]. Brain histology demonstrated a pattern of inflammation, such as microglial activation, astrogliosis, vascular edema, lymphocytic infiltration, neuronal necrosis, neuronophagy, calcifications and apoptosis, which disrupted neural progenitor cells (NPCs) and neurovascular developments [155,156,157]. During ZIKV infection, activated microglia and astrocytes are mainly responsible for the production of several proinflammatory mediators, which are correlated with high expression of apoptosis markers in the brain and leakage of the BBB [155, 156, 158]. The molecular mechanism of ZIKV-mediated inflammation has been explored in vitro and in vivo [158,159,160]. TLR3 signaling was induced by ZIKV infection in both microglia and astrocytes, leading to increased NF-κB and PERK phosphorylation, which triggered the high production of several proinflammatory cytokines involved in the inflammatory process, such as IL-6, IL-1α, IL-4, IL-10, IL-8, monocyte chemoattractant protein-1 (MCP-1), RANTES, IFN-β, and transforming growth factor beta (TGF-β) [158] (Fig. 1). In addition, overproduction of IL-6, macrophage inflammatory protein (MIP)-1α, MIP-1β, MCP-1, TNF-α, IL-1Rα, IL-1α, IL-1β, IL-8, and IL-12p70 was observed in ZIKV-infected primary human fetal brain cells [160]. ZIKV infection induced the activation of NLR family pyrin domain containing 3 (NLRP3) inflammasome in glioblastoma cells [159]. Therefore, the brain damage by uncontrolled inflammation is an important pathogenesis of ZIKV infection.
In addition, in the RABV-infected brain, microglial cells and astrocytes were significantly increased in the areas of the nerve cells that showed apoptosis [100, 103, 161, 162]. The neuronal damage from RABV infection is caused by the high production of inflammatory cytokines in primary astrocytes and microglia [163]. The induction of inflammation in the mouse brain by RABV is observed by the overexpression of TLR3, TLR4, MIP-1α, RANTES, IP-10, MCP-1, TNF-α, and IL-6, which increases RABV pathogenicity [162, 164]. These observations demonstrate that the persistent and high-level expression of chemokines, excessive infiltration, and accumulation of inflammatory cells in the CNS, and severe enhancement of BBB permeability are the major features associated with the neuropathology by neurotropic infection.
Viral Infection Alters Brain Metabolism
Over the past decade, it has become clear that viruses rely on host cell machinery to facilitate their replication. One of these mechanisms is based on the alteration of host cell metabolism, including glycolysis, the pentose phosphate pathway (PPP), the tricarboxylic acid (TCA) cycle, and oxidative phosphorylation, to facilitate optimal viral propagation. In the brain, it is probably more important to acknowledge that virus infection affects neuron and astrocyte metabolism, which disrupts brain function.
Virus Infection Disturbs Neuron Metabolism
Brain development requires a controlled cellular metabolism for neural stem cell proliferation, differentiation, and maturation. Neural stem cell proliferation appears to have a specialized metabolism and is more dependent on glycolysis than oxidative phosphorylation for energy production. In contrast, neuronal differentiation is strongly manipulated by metabolic rewiring from glycolysis to oxidative phosphorylation [165]. Increasing evidence indicates that ZIKV infection suppresses neuron stem cell proliferation and induces premature differentiation, causing microcephaly of the newborn during pregnancy [166,167,168].
In recent publications, a multiomics study comprising the combination of metabolomic data with the information from transcriptomics and proteomics has been used to investigate and model the role of ZIKV-induced microcephaly. It demonstrated the metabolic alterations of nicotinamide adenine dinucleotide (NAD+)-related pathways including the TCA cycle, amino acid metabolism, and mitochondrial oxidative phosphorylation [169]. The mechanisms governing ZIKV disturbance of host cell metabolism are not yet fully understood. An activation of MAPK and cyclic guanidine monophosphate (GMP)-protein kinase G signaling upon ZIKV infection likely has a critical impact on the pathogenesis of ZIKV-induced microcephaly [169]. Initial studies demonstrated that NAD+ metabolism may be highly dependent on MAPK-mediated nicotinamide mononucleotide adenylyltransferase 2 (NMNAT2) degradation, thereby promoting axon degeneration [170, 171]; this suggests that ZIKV infection alters cellular metabolism via the MAPK-NMNAT2-NAD+ axis (Fig. 2). These signatures of metabolic reprogramming are consistent with the findings that ZIKV in both wild-type and mutant strains disrupts glycolytic flow into the TCA cycle, leading to mitochondrial dysfunction, which triggers inflammation and neuronal cell death [172]. In addition, due to the reduction in glycolysis and the TCA cycle seen in ZIKV-infected host cells, there is a dependence on the PPP (Fig. 2). For example, there is actually an increase in metabolic intermediates, such as guanidine diphosphate and GMP, in the PPP of ZIKV-infected cells [172]. Trends toward a decreased glucose contribution to the TCA cycle and an increased contribution to PPP cells to sustain viral propagation were also observed in infected C6/36 mosquitoes [173], suggesting that ZIKV infection in neurons may divert the carbon substrate into the PPP to promote viral replication.
Cellular metabolism in the brain is altered by neurotrophic virus infection. Upon infection, there is a systemic metabolic alteration in neuron and astrocyte. The astrocyte to neuron lactate shuttle is inhibited and subsequently enhanced TCA cycle and oxidative phosphorylation in astrocytes. To fuel active TCA cycle, virus may activate fatty acid β-oxidation and glutamine metabolism. Within the neuron, the metabolic flow of glycolysis, TCA cycle, and oxidative phosphorylation is disrupted and selectively increased pentose phosphate pathway to sustain viral replication. Reduction of lactate production from astrocyte and dysregulation of neuron metabolism cooperatively induce neuron dysfunction. Abbreviation: α-KG, alpha ketoglutarate; β-Ox, beta oxidation; Gln, glutamine; Glu, glutamate; LDH, lactate dehydrogenase; NMN, nicotinamide mononucleotide; PPP, pentose phosphate pathway; OAA, oxaloacetate; OXPHOS, oxidative phosphorylation; SucCoA, succinyl-CoA; TCA, tricarboxylic acid
Virus Infection Disrupts Astrocyte-Neuron Metabolic Cooperation
The metabolism of neurons is functionally linked with astrocytes that provide energetic support to fuel the active neuron. While neuronal cells have robust aerobic glycolysis by converting glucose into acetyl-CoA for the production of substrates for the TCA cycle and oxidative phosphorylation, astrocytes, despite the presence of oxygen, favor the production of lactate. Astrocytes utilize glucose as their main energy source, and approximately 60% of glucose is converted to lactate [174], which is constitutively released to the extracellular milieu and taken up by neuronal cells to supply their high metabolic demand. This cooperative function of astrocytes and neurons is known as the astrocyte-to-neuron lactate shuttle (ANLS) [175]. Astrocyte-neuron metabolic cooperation is tightly regulated, but disrupting this ANLS by viral infection may cause abnormalities in brain function.
Neurological complications in HIV are strongly associated with cognitive impairment. Several studies have shown that induction of viral protein accelerates cellular damage in the CNS. HIV Tat release from infected cells can activate astrocytes and damage surrounding neurons [176, 177]. In fact, a number of studies have provided details about the potential mechanism underlying the role of HIV Tat in astrocyte metabolic shift and neurotoxicity (Fig. 2). First, the decrease in lactate dehydrogenase activity in Tat-activated astrocytes led to a reduction in extracellular lactate levels, which impaired neuronal energy metabolism and function [178]. Second, the cellular response of astrocytes to Tat is a metabolic shift from aerobic glycolysis to mitochondrial respiration mediated by the mitochondrial Ca2+ uniporter (MCU) regulating Ca2+ uptake. Targeting MCU has been found to rescue glycolysis and normalize extracellular lactate levels in astrocytes [178]. Third, astrocyte cellular stress mediated by Tat utilizes fatty acids as the energy source to support mitochondrial respiration [178]. Finally, HIV Tat impacts oxidative injury in astrocytes by reducing glutathione synthase. These changes in turn activate AMPK and increase glycolytic enzymes along with oxidative phosphorylation [179].
Neurological symptoms are more commonly observed in patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2; family Coronaviridae) infection. A number of studies have indicated that the foci of SARS-CoV-2 infection are also observed in astrocytes. The study shows that infected astrocytes alter key proteins and metabolites involved in glycolysis, gluconeogenesis, and the PPP. The decrease in lactate and pyruvate was due to the induction of oxidative metabolism in infected cells [180]. To supply the metabolic intermediates used in oxidative phosphorylation, infected astrocytes tend to alter other metabolic pathways, including glutamine metabolism. Intriguingly, among glutamine metabolic intermediates, glutamine, glutamate, and gamma-aminobutyric acid (GABA) are gradually decreased following SARS-CoV-2 infection [180, 181]. As astrocytes cooperate with neurons to maintain the glutamate-glutamine cycle in the brain, a reduction in glutamine metabolism in astrocytes may shape brain activities.
Virus Infection Affects Neurotransmission System
Previous reports have demonstrated the involvement of viral infection in neuronal dysfunction by affecting neurotransmitter systems. Neurotransmitters are endogenous chemical messengers by which neurons communicate with each other to enable the brain to regulate a variety of functions through the process of synaptic transmission. Alterations in neurotransmitter levels have been observed in some neurotropic viral infections and are correlated with the impairment of specific brain functions. Dopamine is the main catecholamine neurotransmitter that controls voluntary movement, cognition, and endocrine regulation [182]. JEV infection significantly increases dopamine production and modulates the rate-limiting enzyme of dopamine biosynthesis, with an increase in phosphorelated tyrosine hydroxylase levels at the early stage of infection [183]. JEVs exploit dopamine-mediated neuronal communication to increase the susceptibility of dopamine D2 receptors (D2R)-expressing cells to JEV infection, which causes damage to dopaminergic neuron-rich areas such as the thalamus and midbrain, leading to neuronal loss and increasing the fatality rate of JEV-infected mice [183]. The marked decline in catecholamine levels, including norepinephrine, dopamine, 3,4-dihydroxyphenylacetic acid, homovanillic acid, and serotonin, which are unrecoverable, has been reported in the brain, and it leads to the impairment of locomotor activity in JEV-infected rats [184]. The cholinergic system plays an important role in cognitive function. The decrease in acetylcholinesterase (AChE) activity together with damage to different brain regions is associated with the transient dysfunction of learning ability in JEV-infected rats [185, 186]. The decline in muscarinic cholinergic signaling in several brain regions of JEV-infected rats includes the expression levels of cholinergic receptor muscarinic 2 and choline acetyltransferase, and the reduction in total muscarinic cholinergic binding is correlated with transient spatial learning and memory impairment [186].
The balance of glutamate, the most abundant excitatory neurotransmitter, and GABA, an inhibitory neurotransmitter in the CNS, plays a crucial role in several brain functions, including synaptic signaling, cognition, pain, and motor stimuli [187,188,189]. HIV infection causes the imbalance of these two neurotransmitters and is correlated with neuronal and glial dysfunction as well as cognitive impairment in the brains of HIV-seropositive patients with prolonged antiretroviral treatment [190,191,192,193].
Glutamate-mediated excitohypertoxicity is an important mechanism of neuronal injury by viral infection. Several studies have revealed the effect of HIV-1 proteins on neurotoxicity induced by glutamatergic system dysregulation [194]. The HIV-1 Tat protein induces the formation of a macromolecular complex involving N-methyl-d-aspartic acid (NMDA) receptors that promotes apoptosis in neurons and astrocytes [195,196,197]. Dysregulation of glutamate can contribute to HIV-associated neurocognitive disorder [198, 199]. Similar to JEV infection, the increase in glutamate-mediated excitotoxicity activity is correlated with the increase in oxidative stress observed in the brain region responsible for memory and learning impairment in JEV-infected animals [200]. The increased glutamate levels and decreased levels of its NMDA receptors are associated with the activation of TNF-α signaling and the presence of redox imbalance in neural cells, which promote neuronal death [200, 201]. These results suggest that neurotropic virus infection can alter the neurotransmission systems that lead to the dysregulation of brain function and contribute to virus-induced neuropathogenesis.
Virus Infection Accelerates Alzheimer's Disease-Like Pathological Features
Alzheimer’s disease (AD) is the most common cause of dementia that leads to the death of elderly persons. Although age is the most important risk factor for the AD, however, several infections have been suggested to increase the risk of AD. AD is characterized by progressive impairment of synaptic function and degeneration in the brain. Two major pathological features associated with AD include amyloid and tau aggregates in the brain. Amyloid beta (Aβ) plaque accumulation is the main hallmark observed in the brains of AD patients [202]. Aβ is produced by the proteolytic process of the amyloid precursor protein (APP) that occurs at biological membrane. The cleavage of APP by α-secretase or enzymes from the disintegrin and metalloproteinase domain proteins (ADAM) family occurs within the Aβ region on APP; therefore this pathway does not produce Aβ peptide in neurons and called nonamyloidogenic pathway [203]. After α-secretase cleavage, soluble APP α (sAPPα) and α-C-terminal fragment (α-CTF, CTF-83 or C83) are generated. The α-CTF is further cleaved by γ-secretase complex releasing small fragment of extracellular p3 and the amino-terminal APP intracellular domain (AICD) which is rapidly degraded. In nonamyloidogenic pathway, the AICD is rapidly degraded [204]. On the other hand, the amyloidogenic pathway initiates with the cleavage of APP by β-secretase or β-site APP-cleaving enzyme 1 (BACE1), releasing the sAPPβ and leaving the β-C-terminal fragment (β-CTF, CTF-99 or C99) at the membrane. The processing of γ-secretase in this pathway generates two protein products including Aβ and AICD, which are different from another pathway by acting as transcriptional regulators of several target genes. Because γ-secretase can cleave many positions within CTF99, it can create different lengths of Aβ peptide, and the predominant forms are Aβ42 and Aβ40. The insoluble extracellular Aβ aggregates into plaques and can accumulate in the brain [205].
Another neuropathological feature of AD is the accumulation of neurofibrillary tangles (NFTs). NFTs are the result of hyperphosphorylation of the microtubule-stabilizing protein tau. Tau protein is highly expressed in neuronal cells and plays a role in axonal microtubule stabilization, modulation of axonal transport, and neuronal polarity. Tau phosphorylation occurs at serine and threonine sites that are regulated by balancing multiple kinases, such as glycogen synthase kinase 3 beta (GSK-3β) and cyclin-dependent kinase-5 (Cdk-5). Excessive kinase and reduced phosphatase activities cause hyperphosphorylated tau to detach and self-aggregate and cause microtubules to become destabilized [206]. Moreover, GSK-3β, a protein kinase, is not only involved in tau hyperphosphorylation but also linked to other aspects of AD pathogenesis, including Aβ production and mitochondrial dysfunction [207]. Tau deposition in the brain has been suggested to be the consequence of Aβ plaque accumulation [208]. The synergy of Aβ and NFT accumulation is distributed to various neurodegenerative mechanisms, including cholinergic degeneration, synaptic impairment, and neuroinflammation, which are partially responsible for cognitive and behavioral deficits [209].
As demonstrated in several studies, viral infections are related to the induction of pathological hallmarks of AD in association with neuronal damage, synaptic dysfunction, and impairment of cognitive functions. Most of the studies have shown the link between latent viral infections and the risk of developing AD. Latent infections from viruses such as HSV and HIV cause long-term activation of the immune system that leads to chronic neuroinflammation and neurodegeneration in the CNS [210,211,212]. Previous observations reveal a possible indication of HIV and HSV infections as a risk factor associated with AD by distribution of APP and tau-processing homeostasis [213, 214].
The presence of high levels of HSV and its genome have been found in brain tissues, especially in the frontal and temporal cortices and in the CSF of AD patients [211, 215,216,217,218,219]. HSV-1 is a neurotropic virus that lives long within the host and may reactivate after latency and penetrate the BBB into the limbic system and other brain areas that are most often affected in AD [218, 220]. HSV-1 infection causes the alteration of APP processing and has potential causality in the involvement in AD pathogenesis. HSV-1 induces multiple cleavages of APP and promotes intracellular accumulation of various neurotoxic species of Aβ [221,222,223]. The upregulation of BACE1 and the γ-secretase subunit (nicastrin) in the cultured neuronal and glial cells after HSV-1 infection indicates the induction of the amyloidogenic pathway by HSV-1, which leads to a dramatic increase in the intracellular levels of Aβ40 and Aβ42 [224] and in the autophagic compartments [223]. HSV-1 infection modulates the process of autophagy, as shown by the failure of the fusion of autophagosomes containing Aβ with lysosomes, indicating the impaired degradation of Aβ localized in the autophagic vesicles, which contributes to the accumulation of Aβ characteristic of AD [223]. Moreover, HSV-1 infection is associated with the inhibition of the nonamyloidogenic pathway by decreasing α-secretase activity in HSV-1-infected neuroblastoma cells [223]. Oxidative stress induced by HSV-1 potentiates the accumulation of intracellular Aβ and further inhibits its extracellular secretion, which disrupts the autophagic flux in the infected cells [225]. Furthermore, HSV-1 causes functional changes in cortical neurons that induce activity- and Ca2+-mediated APP phosphorylation and intracellular Aβ production [226]. Aβ protein deposits are present in the brains of mice infected with HSV-1 and show cognitive impairment in recurrent HSV-1-infected mice [224, 227]. The induction of APP amyloidogenic processing by HSV-1 also leads to the accumulation and nuclear translocation of AICD in HSV-1-infected neuronal cells [228]. This increase in AICD products in neurons has been observed to bind at the promoter and regulate the transcription of the neprilysin (NEP) gene, whose products are involved in the Aβ clearance process at early stages of infection. In addition, AICD regulates the expression of the GSK3β gene, which plays a role in tau phosphorylation during the late stage of HSV-1 infection [228]. Moreover, the increase in intracellular Aβ accumulation and GSK-3 activation induces synaptic dysfunction, as observed by the reduction in presynaptic proteins (i.e., synapsin-1 and synaptophysin) and the depressed synaptic transmission in cultured cortical neurons infected with HSV-1 [229]. HSV-1 infection affects tau processing by increasing the kinetics of tau aggregation and NFT formation. In the brains of HSV-1-infected mice, p-tau and its cleaved fragments are significantly increased in the cortex and hippocampus compared with noninfected mice [227, 230]. Infection with HSV-1 also induces an increase in tau cleavage, a marker of early neurodegeneration, by the activation of caspase-3 activity in neural cells, which indicates the involvement mechanism of HSVs in the alteration of tau processing homeostasis [227, 231]. In addition, HSV-1 reactivations cause hyperphosphorylation of tau at several sites, including serine 202, threonine 212, serine 214, serine 396, and serine 404, by the induction of GSK-3β and protein kinase A (PKA) activity [229, 231,232,233]. The HSV-1 protein can activate the PI3K/AKT signaling pathway to facilitate viral infection, protein synthesis, and reactivation [234, 235]. The PI3K/AKT pathways are known to regulate GSK-3 activation, which is involved in several cellular functions, including APP and tau hyperphosphorylation [236, 237]. The PI3K/AKT/GSK-3 pathways may be a crucial mechanism involved in the progressive accumulation of AD pathological hallmarks in HSV-1 infection. In the case of herpes simplex virus type 2 (HSV-2) infection, HSV-2 causes a marked accumulation of Aβ40 and Aβ42 in human SK-N-MC neuroblastoma cells [238]. HSV-2 infection decreases the levels of secreted APPα and α-CTF, which indicates the disruption of the APP nonamyloidogenic pathway [238]. Moreover, HSV-2 also induces tau phosphorylation in neuronal cells [238]. Latent infection with HSVs poses a risk of developing AD pathological features in the brain [211].
Epidemiologic studies have implicated the possible involvement of varicella zoster virus (VZV) in AD/dementia [239]. VZV (family Orthoherpesviridae) is an exclusively human neurotropic alphaherpesvirus that causes varicella (chicken pox) upon primary infection after which the virus establishes latency in ganglionic neurons along the entire neuraxis and the reactivation causes zoster (shingles) [240]. VZV causes gliosis and increased levels of several proinflammatory cytokines in human-induced neural stem cells (hiNSC). VZV infection of hiNSCs quiescently infected with HSV-1 leads to HSV-1 reactivation, and the increases in Aβ and p-tau accumulation were observed [239]. This suggests that VZV may reside latently in the brain and, upon reactivation, cause direct damage leading to AD through induced inflammation, which leads to neuroinflammation and reactivation of HSV-1 in the brain and consequent AD-like changes [239]. This report supports the indirect role of VZV in AD/dementia via reactivation of HSV-1 in the brain.
In HIV infection, Aβ deposition is one of the pathologic features found in the brains of HIV patients with prolonged antiretroviral treatment and aging [241, 242]. In long-term HIV infections, the alteration of the accumulation of Aβ42, total tau, and p-tau levels in CSF of HAND cases is similar to that of AD patients [243]. HIV viral proteins may be continually produced, increasing the risk of aged patients developing AD. HIV-1 Tat protein induces Aβ accumulation, tau phosphorylation, and subsequent neuronal death, causing slow cognitive and motor movements, seizures, and premature death [244,245,246,247]. HIV-1 Tat protein reduces clearance of Aβ42 from the brain to the blood and promotes nuclear entry of Aβ as well as inflammatory responses in human brain endothelial cells [248]. Furthermore, HIV-1 Tat protein enhances the cleavage of APP by β-secretase, as shown by the increased levels of β-CTF and reduced levels of α-CTF, resulting in elevated levels of Aβ42 in HIV-1-infected neurons [246, 249]. HIV-1 Tat protein induces impairment of endolysosome structure and function and influences the pathways of Aβ generation, degradation, phagocytosis, and transport, which contribute to HIV-1 neuropathogenesis [249, 250]. Tau processing and HIV-1 Tat protein accelerate tau phosphorylation via multiple mechanisms that lead to the formation of NFTs in HIV transgenic animals [48, 244, 250]. Tau hyperphosphorylation is found in the hippocampus of HIV-1 patients, and high levels of hyperphosphorylated tau are marked in prolonged anti-retroviral therapy-treated subjects [242]. Thus, neurotoxic viral proteins could be a risk factor for brain damage subsequent to AD and/or HIV-associated cognitive impairment.
Viral Infection Induces Proteins to Misfold and Aggregate
The aggregation of aberrantly folded specific proteins is a pathogenic mechanism underlying several neurodegenerative diseases. The misfolded proteins are involved in neurodegenerative diseases including Aβ, p-tau, alpha-synuclein (α-syn), transactive response DNA-binding protein 43 (TDP-43), and prion protein (PrP), share common structural, biological, and biochemical characteristics, as well as similar mechanisms of aggregation and self-propagation. Virus infection induces the deposition of Aβ and p-tau that increases the risk of developing AD, which we have already discussed in the previous section.
Several neuroinvasive viruses have shown a potential relationship to develop PD. PD is a second most common neurodegenerative disorder characterized by progressive loss of dopaminergic neurons of the substantia nigra pars compacta and the presence of Lewy bodies (LBs) together with a reduction of dopamine concentration in the striatum [251]. Abnormal proteinaceous aggregates of α-syn, a major component in LBs, play a causative role in PD pathogenesis [252]. Several studies show that patients with WNV, JEV, DENV, HIV, influenza-A, hepatitis C virus (HCV), hepatitis B virus (HBV), and SARS-CoV-2 infections are at significant risk for developing Parkinson’s symptoms, including tremor, cogwheel rigidity, bradykinesia, and wide gait [253,254,255,256,257,258]. The increase in the amount of α-syn protein found in the primary neurons [259] and in the brains of patients with acute WNV encephalitis [259] and in the brains of HIV patients [260]. HIV-1 Vpr protein triggers the accumulation of α-syn in neurons [261]. Recent in vitro study showed that SARS-CoV-2 spike protein and nucleocapsid protein were found to accelerate the upregulation and aggregation of α-syn, which was associated with the LBs formation [262, 263].
The accumulation of intracellular ubiquitin inclusion bodies in motor neurons in the brain and spinal cord is one of the neuropathologic hallmarks of ALS. TDP-43 is a major component of inclusion bodies in pathological deposits [264, 265]. A number of reports have shown that TDP-43 plays a major role in virus entry, replication, and latency in several viruses [266]. TDP-43 proteinopathy, which is associated with neuronal dysfunction, is induced by several viral infections [266]. The TDP-43 levels were found to increase in the serum of WNV [267] and SARS-CoV-2 [268] patients in association with the high levels of inflammatory markers. Phosphorylated TDP-43 (pTDP-43) inclusion bodies have been found in the brains of SARS-CoV-2 infection [269]. TDP-43 has been shown to be potentially involved in both HIV-1 [270] as well as in HSV-2 [271] latency and cell permittivity, suggesting a possible role for TDP-43 in HIV-1 and HSV-2-associated neurodegeneration in latent infection.
Aggregation and spread of a cellular prion protein (PrPC) throughout the brain also play an important role in the pathogenesis of AD. PrPC is converted into an aggregated neurotoxic isoform called scrapie prion protein (PrPSc) that causes the neuronal death in prion diseases such as Creutzfeldt-Jakob disease in humans, which shared several neuropathological similarities links to AD [272,273,274]. In the hippocampus and temporal cortex of AD brains, PrPC has been shown to co-localize with Aβ plaques [275, 276]. PrPC functions as a receptor for Aβ42 oligomers and mediates the Aβ-induced synaptic dysfunction [277]. The binding of Aβ oligomers to PrPC activates an Aβ-induced signaling cascade involving metabotropic glutamate receptor 5 (mGluR5), tyrosine-protein kinase Fyn, proline-rich tyrosine kinase 2 (Pyk2), and eukaryotic elongation factor 2 kinase (eEF2K) that links Aβ accumulation and tau hyperphosphorylation, resulting in the synaptic failure and neuronal death in the CNS [277,278,279,280,281,282]. PrP expression is upregulated in vitro by infection with a variety of viruses including HCV, HIV-1, human adenovirus type 5, Epstein–Barr virus, murine leukemia virus, and vesicular stomatitis virus [283]. Recent report shows that influenza A virus infection causes PrPC to misfold into PrPSc in mouse neuroblastoma cells [284]. PrPC is significantly elevated in the both neuronal cells and CSF of HIV-1-infected individuals with neurocognitive impairment and mediates neuroinflammation by inducing chemokine release by astrocytes [285]. This evidence suggests that viral infection promotes PrP misfolding and formation of infectious prions in associated with the progression of CNS neurodegeneration, particularly in the pathogenesis of AD.
Thus, viral infection is involved in several specific neurodegeneration-related protein misfolding and subsequent processes of protein aggregate propagation in the CNS leading to neuronal dysfunction, neuroinflammation, and neuronal death that contribute to neurodegenerative disease.
Conclusion
The diagnosis and treatment of CNS infection are urgent needs and challenging. Currently, there are limited or no effective antiviral drugs available for viral infection of the CNS. Neurotropic viruses can cause CNS disease through a variety of molecular mechanisms (Fig. 3), including direct or indirect immune activation, which contributes to the degeneration of neuronal cells. Viral infection promotes an imbalance between free radicals and antioxidants, which increases cellular oxidative stress and leads neuronal cells to undergo programmed cell death by apoptosis. Viruses encounter the cellular recycling process and induce impairment of mitophagy and mitochondrial dynamics in host cells. Dysregulation of mitochondrial homeostasis by viruses affects neuronal metabolism and disrupts brain function. Alterations in neurotransmitter systems and the presence of pathological hallmarks of AD can be observed in neurotropic viral infections in correlation with the specific destruction of brain functions. The fastest protection or cessation of neuronal damage by virus invasion can help to relieve symptoms, which could enhance the survivor rate and reduce severe neurological sequelae. An understanding of the neuropathogenesis of viral CNS infection could support intensive diagnosis and treatment strategies by targeting CNS molecular mechanisms and might be useful for the screening of novel antiviral agents that are essential to improving the management of these neurotropic viral infections.
Data Availability
Not applicable
Abbreviations
- 4-HNE:
-
4-hydroxy-2-nonenal
- AchE:
-
Acetylcholinesterase
- ACOX1:
-
Peroxisomal acyl-coenzyme A oxidase 1
- AD:
-
Alzheimer’s disease
- ADAM:
-
Disintegrin and metalloproteinase domain proteins
- AICD:
-
Amino-terminal APP intracellular domain
- AIDS:
-
Acquired immunodeficiency syndrome
- ALS:
-
Amyotrophic lateral sclerosis
- ANLS:
-
Astrocyte-to-neuron lactate shuttle
- AP-1:
-
Activator protein 1
- APP:
-
Amyloid precursor protein
- ARE:
-
Antioxidant response element
- ATF4:
-
Activating transcription factor 4
- Atg:
-
Autophagy-related gene
- Aβ:
-
Amyloid beta
- BACE1:
-
β-site APP-cleaving enzyme 1
- BAX:
-
BCL-2 associated X
- BBB:
-
Blood–brain barrier
- BCL-2:
-
B-cell lymphoma 2
- Ca2+ :
-
Calcium ion
- CAT:
-
Catalase
- CCL-2:
-
Chemokine (C-C motif) ligand 2
- CCL-5:
-
Chemokine (C-C motif) ligand 5
- CCR5:
-
C-C motif chemokine receptor 5
- Cdk-5:
-
Cyclin-dependent kinase-5
- CDS:
-
Cytosolic DNA sensors
- CHOP:
-
C/EBP homologous protein
- CHPV:
-
Chandipura virus
- CLRs:
-
C-type lectin receptors
- CNS:
-
Central nervous system
- CSF:
-
Cerebrospinal fluid
- CXCL10:
-
C-X-C motif chemokine 10
- CXCR4:
-
C-X-C motif chemokine receptor 4
- CYP2E1:
-
Cytochrome P450-2E1
- Cyt c:
-
Cytochrome c
- D2R:
-
Dopamine D2 receptors
- DENV:
-
Dengue virus
- DENV-2:
-
Dengue virus type 2
- DENV-3:
-
Dengue virus type 3
- DRP1:
-
Dynamin-related protein 1
- eEF2K:
-
Eukaryotic elongation factor 2 kinase
- EGR1:
-
Early growth response 1
- eIF2α:
-
Eukaryotic translation initiation factor 2α kinase
- ER:
-
endoplasmic reticulum
- ERK:
-
Extracellular-signal-regulated kinase
- ETC:
-
Electron transport chain
- EV71:
-
Enterovirus 71
- GABA:
-
Gamma-aminobutyric acid
- GADD153:
-
Growth arrest and DNA damage-inducible protein
- GMP:
-
Cyclic guanidine monophosphate
- GPx:
-
Glutathione peroxidase
- GR:
-
Glutathione reductase
- GRP78:
-
Glucose-regulated protein 78 kDa
- GSH:
-
Glutathione
- GSK-3β:
-
Glycogen synthase kinase 3 beta
- HAEs:
-
Hydroxyalkenals
- HANDs:
-
HIV-associated neurocognitive disorders
- HBV:
-
Hepatitis B virus
- HCV:
-
Hepatitis C virus
- HD:
-
Huntington’s disease
- HFMD:
-
Hand, foot, and mouth disease
- HIV:
-
Human immunodeficiency virus
- HSP:
-
Heat shock protein
- HSV:
-
Herpes simplex virus
- HSV-1:
-
Herpes simplex virus type 1
- HSV-2:
-
Herpes simplex virus type 2
- IFNγ:
-
Interferon gamma
- IL:
-
Interleukin
- iNOS:
-
Inducible nitric oxide synthase
- JEV:
-
Japanese encephalitis virus
- LBs:
-
Lewy bodies
- LC3B:
-
Microtubule-associated protein 1 light chain 3 beta
- MAPK:
-
Mitogen-activated protein kinase
- MCP-1:
-
Monocyte chemoattractant protein-1
- MCU:
-
Mitochondrial Ca2+ uniporter
- MDA:
-
Malondialdehyde
- mGluR5:
-
Metabotropic glutamate receptor 5
- MIP-1α:
-
Macrophage inflammatory protein 1 alpha
- MOI:
-
Multiplicity of infection
- MPR:
-
Mannose-6-phosphate receptor
- mTOR:
-
Mammalian target of rapamycin
- NAD+:
-
Nicotinamide adenine dinucleotide
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate
- NFTs:
-
Neurofibrillary tangles
- NF-κB:
-
Nuclear factor kappa B
- NLRP3:
-
NLR family pyrin domain containing 3
- NLRs:
-
NOD-like receptors
- NMDA:
-
N-methyl-d-aspartic acid
- NMNAT2:
-
MAPK-mediated nicotinamide mononucleotide adenylyltransferase 2
- NO:
-
Nitric oxide
- NPCs:
-
Neural progenitor cells
- NRF2:
-
Nuclear factor erythroid 2-related factor 2
- NS4B:
-
Nonstructural protein 4B
- O2.-:
-
Superoxide anions
- OONO-:
-
Peroxynitrite
- p70S6K:
-
Phosphorylated 70-kDa S6 kinase
- PARP:
-
Poly ADP-ribose polymerase
- PD:
-
Parkinson’s disease
- PERK:
-
Protein kinase RNA-like ER kinase
- PET:
-
Positron emission tomography
- PINK1:
-
PTEN-induced kinase 1
- PKA:
-
Protein kinase A
- PMP22:
-
Peripheral myelin protein 22
- PPP:
-
Pentose phosphate pathway
- PrP:
-
Prion protein
- PrPC :
-
Cellular prion protein
- PrPSc :
-
Scrapie prion protein
- PRRs:
-
Pattern recognition receptors
- pTDP-43:
-
Phosphorylated TDP-43
- Pyk2:
-
Proline-rich tyrosine kinase 2
- RABV:
-
Rabies virus
- RANTES:
-
Regulated on activation, normal T-cell expressed, and secreted
- RIG-I:
-
Retinoic acid-inducible gene I
- RLRs:
-
Retinoic acid-inducible gene-I-like receptors
- ROS:
-
Reactive oxygen species
- sAPPα:
-
Soluble APP alpha
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- Ser51:
-
Serine51
- SOD-1:
-
Superoxide dismutase 1
- SQSTM1:
-
Sequestosome 1
- TBARS :
-
Thiobarbituric acid reactive substances
- TBEV:
-
Tick-borne encephalitis virus
- TCA:
-
Tricarboxylic acid cycle
- TDP-43 :
-
Transactive response DNA-binding protein 43
- TGF-β:
-
Transforming growth factor beta
- TLR:
-
Toll-like receptor
- TNF-α:
-
Tumor necrosis factor alpha
- TNF-β:
-
Tumor necrosis factor beta
- TRADD:
-
TNF receptor-associated death domain
- TRX-1:
-
Thioredoxin 1
- TSPO:
-
Translocator protein
- UPR:
-
Unfolded protein response
- VEEV:
-
Venezuelan equine encephalitis virus
- VP1:
-
Viral envelope protein 1
- VZV:
-
Varicella zoster virus
- WNV:
-
West Nile virus
- ZIKV:
-
Zika virus
- α-CTF:
-
α-C-terminal fragment
- α-syn:
-
Alpha-synuclein
- β-CTF:
-
β-C-terminal fragment
References
Przedborski S, Vila M, Jackson-Lewis V (2003) Neurodegeneration: what is it and where are we? J Clin Invest 111:3–10
Li PA, Hou X, Hao S (2017) Mitochondrial biogenesis in neurodegeneration. J Neurosci Res 95:2025–2029
Jellinger KA (2010) Basic mechanisms of neurodegeneration: a critical update. J Cell Mol Med 14:457–487
Moujalled D, Strasser A, Liddell JR (2021) Molecular mechanisms of cell death in neurological diseases. Cell Death Differ 28:2029–2044
Kwon HS, Koh SH (2020) Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes. Transl Neurodegener 9:42
Wongchitrat P, Shukla M, Sharma R, Govitrapong P, Reiter RJ (2021) Role of melatonin on virus-induced neuropathogenesis-a concomitant therapeutic strategy to understand SARS-CoV-2 infection. Antioxidants (Basel) 10:47
Singh H, Koury J, Kaul M (2021) Innate immune sensing of viruses and its consequences for the central nervous system. Viruses 13:170
Feige L, Zaeck LM, Sehl-Ewert J, Finke S, Bourhy H (2021) Innate immune signaling and role of glial cells in herpes simplex virus- and rabies virus-induced encephalitis. Viruses 13:2364
Cohen FS (2016) How Viruses Invade Cells. Biophys J 110:1028–1032
Kigerl KA, de Rivero Vaccari JP, Dietrich WD, Popovich PG, Keane RW (2014) Pattern recognition receptors and central nervous system repair. Exp Neurol 258:5–16
Zhou L, Miranda-Saksena M, Saksena NK (2013) Viruses and neurodegeneration. Virol J 10:172
Amor S, Puentes F, Baker D, van der Valk P (2010) Inflammation in neurodegenerative diseases. Immunology 129:154–169
Unni SK et al (2011) Japanese encephalitis virus: from genome to infectome. Microbes Infect 13:312–321
Fischer M, Lindsey N, Staples JE, Hills S (2010) Japanese encephalitis vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 59:1–27
Lin RJ, Liao CL, Lin YL (2004) Replication-incompetent virions of Japanese encephalitis virus trigger neuronal cell death by oxidative stress in a culture system. J Gen Virol 85:521–533
Mishra MK, Ghosh D, Duseja R, Basu A (2009) Antioxidant potential of minocycline in Japanese encephalitis virus infection in murine neuroblastoma cells: correlation with membrane fluidity and cell death. Neurochem Int 54:464–470
Srivastava R, Kalita J, Khan MY, Misra UK (2009) Free radical generation by neurons in rat model of Japanese encephalitis. Neurochem Res 34:2141–2146
Raung SL, Kuo MD, Wang YM, Chen CJ (2001) Role of reactive oxygen intermediates in Japanese encephalitis virus infection in murine neuroblastoma cells. Neurosci Lett 315:9–12
Lixia H, Jun C, Song H, FaHu Y, Jinwen T (2018) Neuroprotective effect of (-)-tetrahydropalmatine in Japanese encephalitis virus strain GP-78 infected mouse model. Microb Pathog 114:197–203
Verma S et al (2006) Role of oxidative stress in West Nile virus (WNV)-induced apoptosis. FASEB J 20:A1073–A1073
Jan JT et al (2000) Potential dengue virus-triggered apoptotic pathway in human neuroblastoma cells: arachidonic acid, superoxide anion, and NF-kappaB are sequentially involved. J Virol 74:8680–8691
Weaver SC et al (1996) Re-emergence of epidemic Venezuelan equine encephalomyelitis in South America. VEE Study Group. Lancet 348:436–440
Bowen GS, Fashinell TR, Dean PB, Gregg MB (1976) Clinical aspects of human Venezuelan equine encephalitis in Texas. Bull Pan Am Health Organ 10:46–57
Ronca SE, Dineley KT, Paessler S (2016) Neurological sequelae resulting from encephalitic alphavirus infection. Front Microbiol 7:959
Keck F et al (2017) Altered mitochondrial dynamics as a consequence of Venezuelan Equine encephalitis virus infection. Virulence 8:1849–1866
Valero N, MarinaEspina L, Bonilla E, Mosquera J (2007) Melatonin decreases nitric oxide production and lipid peroxidation and increases interleukin-1 beta in the brain of mice infected by the Venezuelan equine encephalomyelitis virus. J Pineal Res 42:107–112
Kumar S, Misra UK, Kalita J, Khanna VK, Khan MY (2009) Imbalance in oxidant/antioxidant system in different brain regions of rat after the infection of Japanese encephalitis virus. Neurochem Int 55:648–654
Bourhy H, Gubala AJ, Weir RP, Boyle DB (2008) Animal Rhabdoviruses. In: Mahy BWJ, Van Regenmortel MHV (eds) Encyclopedia of Virology, Third edn. Academic Press, Oxford, pp. 111–121. https://doi.org/10.1016/B978-012374410-4.00783-4
Kammouni W et al (2015) Rabies virus phosphoprotein interacts with mitochondrial Complex I and induces mitochondrial dysfunction and oxidative stress. J Neurovirol 21:370–382
Kammouni W, Wood H, Jackson AC (2017) Serine residues at positions 162 and 166 of the rabies virus phosphoprotein are critical for the induction of oxidative stress in rabies virus infection. J Neurovirol 23:358–368
Jackson AC, Kammouni W, Zherebitskaya E, Fernyhough P (2010) Role of oxidative stress in rabies virus infection of adult mouse dorsal root ganglion neurons. J Virol 84:4697–4705
Verma AK, Ghosh S, Basu A (2018) Chandipura Virus induced neuronal apoptosis via calcium signaling mediated oxidative stress. Front Microbiol 9:1489
Ghosh S, Dutta K, Basu A (2013) Chandipura virus induces neuronal death through Fas-mediated extrinsic apoptotic pathway. J Virol 87:12398–12406
Ghosh S et al (2016) Network analysis reveals common host protein/s modulating pathogenesis of neurotropic viruses. Sci Rep 6:32593
Cheng ML, Weng SF, Kuo CH, Ho HY (2014) Enterovirus 71 induces mitochondrial reactive oxygen species generation that is required for efficient replication. PLoS One 9:e113234
You L et al (2020) Enterovirus 71 induces neural cell apoptosis and autophagy through promoting ACOX1 downregulation and ROS generation. Virulence 11:537–553
Tung WH, Hsieh HL, Lee IT, Yang CM (2011) Enterovirus 71 induces integrin beta1/EGFR-Rac1-dependent oxidative stress in SK-N-SH cells: role of HO-1/CO in viral replication. J Cell Physiol 226:3316–3329
Rozenberg F (2013) Chapter 122 - Acute viral encephalitis. In: Dulac O, Lassonde M, Sarnat HB (eds) Handbook of Clinical Neurology, vol 112. (Elsevier, pp. 1171–1181
Hu S, Sheng WS, Schachtele SJ, Lokensgard JR (2011) Reactive oxygen species drive herpes simplex virus (HSV)-1-induced proinflammatory cytokine production by murine microglia. J Neuroinflammation 8:123
Kavouras JH et al (2007) Herpes simplex virus type 1 infection induces oxidative stress and the release of bioactive lipid peroxidation by-products in mouse P19N neural cell cultures. J Neurovirol 13:416–425
Woods SP, Moore DJ, Weber E, Grant I (2009) Cognitive neuropsychology of HIV-associated neurocognitive disorders. Neuropsychol Rev 19:152–168
Silverstein PS et al (2012) HIV-1 gp120 and drugs of abuse: interactions in the central nervous system. Curr HIV Res 10:369–383
Kaul M, Zheng J, Okamoto S, Gendelman HE, Lipton SA (2005) HIV-1 infection and AIDS: consequences for the central nervous system. Cell Death Differ 12(Suppl 1):878–892
Guo L et al (2013) Curcumin protects microglia and primary rat cortical neurons against HIV-1 gp120-mediated inflammation and apoptosis. PLoS One 8:e70565
Shah A, Kumar S, Simon SD, Singh DP, Kumar A (2013) HIV gp120- and methamphetamine-mediated oxidative stress induces astrocyte apoptosis via cytochrome P450 2E1. Cell Death Dis 4:e850
Ivanov AV et al (2016) Oxidative stress during HIV infection: mechanisms and consequences. Oxid Med Cell Longev 2016:8910396
Rozzi SJ et al (2014) PACAP27 is protective against tat-induced neurotoxicity. J Mol Neurosci 54:485–493
Cho YE, Lee MH, Song BJ (2017) Neuronal cell death and degeneration through increased nitroxidative stress and tau phosphorylation in HIV-1 transgenic rats. PLoS One 12:e0169945
Munoz LS, Parra B, Pardo CA, S. (2017) Neuroviruses emerging in the Americas, neurological implications of Zika virus infection in adults. J Infect Dis 216:S897–S905
Acosta-Ampudia Y et al (2018) Autoimmune neurological conditions associated with Zika virus infection. Front Mol Neurosci 11:116
Ledur PF et al (2020) Zika virus infection leads to mitochondrial failure, oxidative stress and DNA damage in human iPSC-derived astrocytes. Sci Rep 10:1218
Almeida LT et al (2020) Zika virus induces oxidative stress and decreases antioxidant enzyme activities in vitro and in vivo. Virus Res 286:198084
Camini FC, da Silva Caetano CC, Almeida LT, de Brito Magalhaes CL (2017) Implications of oxidative stress on viral pathogenesis. Arch Virol 162:907–917
Deramaudt TB, Dill C, Bonay M (2013) Regulation of oxidative stress by Nrf2 in the pathophysiology of infectious diseases. Med Mal Infect 43:100–107
Ho H-Y et al (2008) Glucose-6-phosphate dehydrogenase deficiency enhances enterovirus 71 infection. J Gen Virol 89:2080–2089
Nucci C et al (2000) Imbalance in corneal redox state during herpes simplex virus 1-induced keratitis in rabbits. Effectiveness of Exogenous Glutathione Supply. Exp Eye Res 70:215–220
Price TO, Ercal N, Nakaoke R, Banks WA (2005) HIV-1 viral proteins gp120 and Tat induce oxidative stress in brain endothelial cells. Brain Res 1045:57–63
Alandijany T, Kammouni W, Roy Chowdhury SK, Fernyhough P, Jackson AC (2013) Mitochondrial dysfunction in rabies virus infection of neurons. J NeuroVirology 19:537–549
Scott CA, Rossiter JP, Andrew RD, Jackson AC (2008) Structural abnormalities in neurons are sufficient to explain the clinical disease and fatal outcome of experimental rabies in yellow fluorescent protein-expressing transgenic mice. J Virol 82:513–521
Jackson AC, Kammouni W, Fernyhough P (2011) Chapter 8 - Role of Oxidative Stress in Rabies Virus Infection. In: Jackson AC (ed) Advances in Virus Research, vol 79. (Academic Press, pp. 127–138
Kaufman RJ (1999) Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev 13:1211–1233
Zeeshan HM, Lee GH, Kim HR, Chae HJ (2016) Endoplasmic reticulum stress and associated ROS. Int J Mol Sci 17:327
Ghosh Roy S, Sadigh B, Datan E, Lockshin RA, Zakeri Z (2014) Regulation of cell survival and death during Flavivirus infections. World. J Biol Chem 5:93–105
Mukherjee S et al (2018) Japanese encephalitis virus induces human neural stem/progenitor cell death by elevating GRP78, PHB and hnRNPC through ER stress. Cell Death Dis 8:e2556–e2556
Wang Q et al (2019) Japanese encephalitis virus induces apoptosis and encephalitis by activating the PERK pathway. J Virol 93:e00887–e00819
Medigeshi GR et al (2007) West Nile virus infection activates the unfolded protein response, leading to CHOP induction and apoptosis. J Virol 81:10849–10860
Baer A et al (2016) Venezuelan equine encephalitis virus induces apoptosis through the unfolded protein response activation of EGR1. J Virol 90:3558–3572
Dahal B et al (2020) EGR1 upregulation following Venezuelan equine encephalitis virus infection is regulated by ERK and PERK pathways contributing to cell death. Virology 539:121–128
Tan Z et al (2018) ZIKV infection activates the IRE1-XBP1 and ATF6 pathways of unfolded protein response in neural cells. J Neuroinflammation 15:275
Gladwyn-Ng I et al (2018) Stress-induced unfolded protein response contributes to Zika virus-associated microcephaly. Nat Neurosci 21:63–71
Hu DD et al (2017) Glucocorticoids prevent enterovirus 71 capsid protein VP1 induced calreticulin surface exposure by alleviating neuronal ER stress. Neurotox Res 31:204–217
Li P-Q et al (2018) Enterovirus 71 structural viral protein 1 promotes mouse Schwann cell autophagy via endoplasmic reticulum stress-mediated peripheral myelin protein 22 upregulation. bioRxiv 10(1101/314468):314468
Mehrbod P et al (2019) The roles of apoptosis, autophagy and unfolded protein response in arbovirus, influenza virus, and HIV infections. Virulence 10:376–413
Lee YR, Wang PS, Wang JR, Liu HS (2014) Enterovirus 71-induced autophagy increases viral replication and pathogenesis in a suckling mouse model. J Biomed Sci 21:80
Huang SC, Chang CL, Wang PS, Tsai Y, Liu HS (2009) Enterovirus 71-induced autophagy detected in vitro and in vivo promotes viral replication. J Med Virol 81:1241–1252
Liu ZW et al (2019) Enterovirus 71 VP1 protein regulates viral replication in SH-SY5Y cells via the mTOR autophagy signaling pathway. Viruses 12:11
Too IH et al (2016) Enterovirus 71 infection of motor neuron-like NSC-34 cells undergoes a non-lytic exit pathway. Sci Rep 6:36983
Zhou D, Masliah E, Spector SA (2011) Autophagy is increased in postmortem brains of persons with HIV-1-associated encephalitis. J Infect Dis 203:1647–1657
Fields JA et al (2017) The anticancer drug sunitinib promotes autophagyand protects from neurotoxicity in an HIV-1 Tat model of neurodegeneration. J Neurovirol 23:290–303
Cheney L et al (2020) HIV Nef and antiretroviral therapy have an inhibitory effect on autophagy in human astrocytes that may contribute to HIV-associated neurocognitive disorders. Cells 9:1426
Fields J et al (2015) HIV-1 Tat alters neuronal autophagy by modulating autophagosome fusion to the lysosome: implications for HIV-associated neurocognitive disorders. J Neurosci 35:1921–1938
Jin R et al (2013) Japanese encephalitis virus activates autophagy as a viral immune evasion strategy. PLoS One 8:e52909
Zhang L, Qin Y, Chen M (2018) Viral strategies for triggering and manipulating mitophagy. Autophagy 14:1665–1673
Teodorof-Diedrich C, Spector SA (2018) Human immunodeficiency virus type 1 gp120 and Tat induce mitochondrial fragmentation and incomplete mitophagy in human neurons. J Virol 92:e00993-18
Teodorof-Diedrich C, Spector SA (2020) Human immunodeficiency virus type-1 and methamphetamine mediated mitochondrial damage and neuronal degeneration in human neurons. J Virol. https://doi.org/10.1128/JVI.00924-20
Rozzi SJ, Avdoshina V, Fields JA, Mocchetti I (2018) Human immunodeficiency virus Tat impairs mitochondrial fission in neurons. Cell Death Discov 4:8
Thangaraj A et al (2018) HIV-1 TAT-mediated microglial activation: role of mitochondrial dysfunction and defective mitophagy. Autophagy 14:1596–1619
Tsao CH et al (2008) Japanese encephalitis virus infection activates caspase-8 and -9 in a FADD-independent and mitochondrion-dependent manner. J Gen Virol 89:1930–1941
Swarup V, Das S, Ghosh S, Basu A (2007) Tumor necrosis factor receptor-1-induced neuronal death by TRADD contributes to the pathogenesis of Japanese encephalitis. J Neurochem 103:771–783
James HJ et al (1999) Expression of caspase-3 in brains from paediatric patients with HIV-1 encephalitis. Neuropathol Appl Neurobiol 25:380–386
Lannuzel A et al (1997) Human immunodeficiency virus type 1 and its coat protein gp120 induce apoptosis and activate JNK and ERK mitogen-activated protein kinases in human neurons. Ann Neurol 42:847–856
Zhang Y et al (2012) Sigma-1 receptor agonists provide neuroprotection against gp120 via a change in bcl-2 expression in mouse neuronal cultures. Brain Res 1431:13–22
Chandrasekar AP, Cummins NW, Badley AD (2019) The role of the BCL-2 family of proteins in HIV-1 pathogenesis and persistence. Clin Microbiol Rev 33:e00107-19
Jackson AC, Rossiter JP (1997) Apoptotic cell death is an important cause of neuronal injury in experimental Venezuelan equine encephalitis virus infection of mice. Acta Neuropathol 93:349–353
Bocan TM et al (2019) Characterization of brain inflammation, apoptosis, hypoxia, blood-brain barrier integrity and metabolism in Venezuelan equine encephalitis virus (VEEV TC-83) exposed mice by in vivo positron emission tomography imaging. Viruses 11:1052
Du X et al (2015) Enterovirus 71 induces apoptosis of SHSY5Y human neuroblastoma cells through stimulation of endogenous microRNA let-7b expression. Mol Med Rep 12:953–959
Wang C et al (2015) Intrinsic apoptosis and proinflammatory cytokines regulated in human astrocytes infected with enterovirus 71. J Gen Virol 96:3010–3022
Luo Z et al (2019) EV71 infection induces neurodegeneration via activating TLR7 signaling and IL-6 production. PLoS Pathog 15:e1008142
Jackson AC, Rossiter JP (1997) Apoptosis plays an important role in experimental rabies virus infection. J Virol 71:5603–5607
Rutherford M, Jackson AC (2004) Neuronal apoptosis in immunodeficient mice infected with the challenge virus standard strain of rabies virus by intracerebral inoculation. J Neurovirol 10:409–413
Jackson AC (1999) Apoptosis in experimental rabies in bax-deficient mice. Acta Neuropathol 98:288–294
Fu ZF, Jackson AC (2005) Neuronal dysfunction and death in rabies virus infection. J Neurovirol 11:101–106
Kojima D et al (2010) Lesions of the central nervous system induced by intracerebral inoculation of BALB/c mice with rabies virus (CVS-11). J Vet Med Sci 72:1011–1016
Sevil Atalay V, Mehmet Fatih B, Ali O, Mehmet Eray A, Fatma Sayin I (2016) Apoptosis in natural rabies virus infection in dogs. Journal of. Vet Res 60:227–231
Kojima D et al (2009) Pathology of the spinal cord of C57BL/6J mice infected with rabies virus (CVS-11 strain). J Vet Med Sci 71:319–324
Ubol S, Sukwattanapan C, Utaisincharoen P (1998) Rabies virus replication induces Bax-related, caspase dependent apoptosis in mouse neuroblastoma cells. Virus Res 56:207–215
Parquet MC, Kumatori A, Hasebe F, Morita K, Igarashi A (2001) West Nile virus-induced bax-dependent apoptosis. FEBS Lett 500:17–24
Kleinschmidt MC, Michaelis M, Ogbomo H, Doerr HW, Cinatl J Jr (2007) Inhibition of apoptosis prevents West Nile virus induced cell death. BMC Microbiol 7:49
van Marle G et al (2007) West Nile virus-induced neuroinflammation: glial infection and capsid protein-mediated neurovirulence. J Virol 81:10933–10949
Yang JS et al (2002) Induction of inflammation by West Nile virus capsid through the caspase-9 apoptotic pathway. Emerg Infect Dis 8:1379–1384
Ramanathan MP et al (2006) Host cell killing by the West Nile virus NS2B-NS3 proteolytic complex: NS3 alone is sufficient to recruit caspase-8-based apoptotic pathway. Virology 345:56–72
Chu JJH, Ng ML (2003) The mechanism of cell death during West Nile virus infection is dependent on initial infectious dose. J Gen Virol 84:3305–3314
Samuel MA, Morrey JD, Diamond MS (2007) Caspase 3-dependent cell death of neurons contributes to the pathogenesis of West Nile virus encephalitis. J Virol 81:2614–2623
Peng BH, Wang T (2019) West Nile Virus Induced Cell Death in the Central Nervous System. Pathogens 8:215
Mishra MK, Basu A (2008) Minocycline neuroprotects, reduces microglial activation, inhibits caspase 3 induction, and viral replication following Japanese encephalitis. J Neurochem 105:1582–1595
Kitidee K et al (2023) Antiviral effect of melatonin on Japanese encephalitis virus infection involves inhibition of neuronal apoptosis and neuroinflammation in SH-SY5Y cells. Sci Rep 13:6063
Wongchitrat P et al (2019) Elevation of cleaved p18 Bax levels associated with the kinetics of neuronal cell death during Japanese encephalitis virus infection. Int J Mol Sci 20:5016
Al-Obaidi MMJ et al (2017) Japanese encephalitis virus disrupts blood-brain barrier and modulates apoptosis proteins in THBMEC cells. Virus Res 233:17–28
Okamoto T et al (2017) Regulation of apoptosis during flavivirus infection. Viruses 9:243
Lee CJ, Liao CL, Lin YL (2005) Flavivirus activates phosphatidylinositol 3-kinase signaling to block caspase-dependent apoptotic cell death at the early stage of virus infection. J Virol 79:8388–8399
Liao C-L et al (1998) Antiapoptotic but not antiviral function of human bcl-2 assists establishment of Japanese encephalitis virus persistence in cultured cells. J Virol 72:9844
Liao CL et al (1997) Effect of enforced expression of human bcl-2 on Japanese encephalitis virus-induced apoptosis in cultured cells. J Virol 71:5963–5971
Gao G, Dou QP (2001) N-terminal cleavage of Bax by calpain generates a potent proapoptotic 18-kDa fragment that promotes Bcl-2-independent cytochrome C release and apoptotic cell death. J Cell Biochem 80:53–72
Klein RS, Garber C, Howard N (2017) Infectious immunity in the central nervous system and brain function. Nat Immunol 18:132–141
Lannes N, Summerfield A, Filgueira L (2017) Regulation of inflammation in Japanese encephalitis. J Neuroinflammation 14:158
Hsieh JT, Rathore APS, Soundarajan G, St John AL (2019) Japanese encephalitis virus neuropenetrance is driven by mast cell chymase. Nat Commun 10:706
Lannes N et al (2017) Interactions of human microglia cells with Japanese encephalitis virus. Virol J 14:8
Ghoshal A et al (2007) Proinflammatory mediators released by activated microglia induces neuronal death in Japanese encephalitis. Glia 55:483–496
Jiang R et al (2014) Roles of TLR3 and RIG-I in mediating the inflammatory response in mouse microglia following Japanese encephalitis virus infection. J Immunol Res 2014:787023
Chen CJ et al (2010) Glial activation involvement in neuronal death by Japanese encephalitis virus infection. J Gen Virol 91:1028–1037
Ye J et al (2014) Etanercept reduces neuroinflammation and lethality in mouse model of Japanese encephalitis. J Infect Dis 210:875–889
Niranjan R, Muthukumaravel S, Jambulingam P (2019) The involvement of neuroinflammation in dengue viral disease: importance of innate and adaptive immunity. Neuroimmunomodulation 26:111–118
Al-Shujairi WH et al (2017) Intracranial injection of dengue virus induces interferon stimulated genes and CD8+ T cell infiltration by sphingosine kinase 1 independent pathways. PLoS One 12:e0169814
Amaral DC et al (2011) Intracerebral infection with dengue-3 virus induces meningoencephalitis and behavioral changes that precede lethality in mice. J Neuroinflammation 8:23
Han YW et al (2014) Distinct dictation of Japanese encephalitis virus-induced neuroinflammation and lethality via triggering TLR3 and TLR4 signal pathways. PLoS Pathog 10:e1004319
Li F et al (2015) Viral Infection of the central nervous system and neuroinflammation precede blood-brain barrier disruption during Japanese encephalitis virus infection. J Virol 89:5602–5614
Das S, Mishra MK, Ghosh J, Basu A (2008) Japanese encephalitis virus infection induces IL-18 and IL-1beta in microglia and astrocytes: correlation with in vitro cytokine responsiveness of glial cells and subsequent neuronal death. J Neuroimmunol 195:60–72
Keck F et al (2018) Mitochondrial-directed antioxidant reduces microglial-induced inflammation in murine in vitro model of TC-83 infection. Viruses 10:606
Lin TY, Hsia SH, Huang YC, Wu CT, Chang LY (2003) Proinflammatory cytokine reactions in enterovirus 71 infections of the central nervous system. Clin Infect Dis 36:269–274
Wang SM et al (2007) Cerebrospinal fluid cytokines in enterovirus 71 brain stem encephalitis and echovirus meningitis infections of varying severity. Clin Microbiol Infect 13:677–682
Wong KT et al (2008) The distribution of inflammation and virus in human enterovirus 71 encephalomyelitis suggests possible viral spread by neural pathways. J Neuropathol Exp Neurol 67:162–169
Zhang Y et al (2011) Pathogenesis study of enterovirus 71 infection in rhesus monkeys. Lab Invest 91:1337–1350
Chang CY et al (2015) Enterovirus 71 infection caused neuronal cell death and cytokine expression in cultured rat neural cells. IUBMB Life 67:789–800
Bogovic P, Strle F (2015) Tick-borne encephalitis: A review of epidemiology, clinical characteristics, and management. World J Clin Cases 3:430–441
Zajkowska J et al (2011) Evaluation of CXCL10, CXCL11, CXCL12 and CXCL13 chemokines in serum and cerebrospinal fluid in patients with tick borne encephalitis (TBE). Adv Med Sci 56:311–317
Grygorczuk S et al (2015) Increased concentration of interferon lambda-3, interferon beta and interleukin-10 in the cerebrospinal fluid of patients with tick-borne encephalitis. Cytokine 71:125–131
Bardina SV, Lim JK (2012) The role of chemokines in the pathogenesis of neurotropic flaviviruses. Immunol Res 54:121–132
Palus M et al (2015) Analysis of serum levels of cytokines, chemokines, growth factors, and monoamine neurotransmitters in patients with tick-borne encephalitis: identification of novel inflammatory markers with implications for pathogenesis. J Med Virol 87:885–892
Palus M et al (2017) Tick-borne encephalitis virus infects human brain microvascular endothelial cells without compromising blood-brain barrier integrity. Virology 507:110–122
Selinger M et al (2022) Integrative RNA profiling of TBEV-infected neurons and astrocytes reveals potential pathogenic effectors. Comput Struct Biotechnol J 20:2759–2777
Potokar M, Korva M, Jorgacevski J, Avsic-Zupanc T, Zorec R (2014) Tick-borne encephalitis virus infects rat astrocytes but does not affect their viability. PLoS One 9:e86219
Fares M et al (2020) Pathological modeling of TBEV infection reveals differential innate immune responses in human neurons and astrocytes that correlate with their susceptibility to infection. J Neuroinflammation 17:76
Pokorna Formanova P et al (2019) Changes in cytokine and chemokine profiles in mouse serum and brain, and in human neural cells, upon tick-borne encephalitis virus infection. J Neuroinflammation 16:205
Zhang X et al (2016) Tick-borne encephalitis virus induces chemokine RANTES expression via activation of IRF-3 pathway. J Neuroinflammation 13:209
Shao Q et al (2016) Zika virus infection disrupts neurovascular development and results in postnatal microcephaly with brain damage. Development 143:4127–4136
de Sousa JR et al (2018) Correlation between apoptosis and in situ immune response in fatal cases of microcephaly caused by Zika virus. Am J Pathol 188:2644–2652
Panganiban AT et al (2020) A Zika virus primary isolate induces neuroinflammation, compromises the blood-brain barrier, and upregulates CXCL12 in adult macaques. Brain Pathol. https://doi.org/10.1111/bpa.12873
Ojha CR et al (2019) Toll-like receptor 3 regulates Zika virus infection and associated host inflammatory response in primary human astrocytes. PLoS One 14:e0208543
Tricarico PM, Caracciolo I, Crovella S, D'Agaro P (2017) Zika virus induces inflammasome activation in the glial cell line U87-MG. Biochem Biophys Res Commun 492:597–602
Lum FM et al (2017) Zika virus infects human fetal brain microglia and induces inflammation. Clin Infect Dis 64:914–920
Shuangshoti S et al (2013) Reduced viral burden in paralytic compared to furious canine rabies is associated with prominent inflammation at the brainstem level. BMC Vet Res 9:31
Farahtaj F et al (2019) Natural infection with rabies virus: a histopathological and immunohistochemical study of human brains. Osong Public Health Res Perspect 10:6–11
Li Y et al (2020) Interferon-lambda attenuates rabies virus infection by inducing interferon-stimulated genes and alleviating neurological inflammation. Viruses 12:405
Zhao L, Toriumi H, Kuang Y, Chen H, Fu ZF (2009) The roles of chemokines in rabies virus infection: overexpression may not always be beneficial. J Virol 83:11808–11818
Iwata R, Vanderhaeghen P (2021) Regulatory roles of mitochondria and metabolism in neurogenesis. Curr Opin Neurobiol 69:231–240
Li C et al (2016) Zika virus disrupts neural progenitor development and leads to microcephaly in mice. Cell Stem Cell 19:120–126
Tiwari SK et al (2020) Zika virus depletes neural stem cells and evades selective autophagy by suppressing the Fanconi anemia protein FANCC. EMBO Rep 21:e49183
Gabriel E et al (2017) Recent Zika virus isolates induce premature differentiation of neural progenitors in human brain organoids. Cell Stem Cell 20:397–406 e395
Pang H et al (2021) Aberrant NAD(+) metabolism underlies Zika virus-induced microcephaly. Nat Metab 3:1109–1124
Walker LJ et al (2017) MAPK signaling promotes axonal degeneration by speeding the turnover of the axonal maintenance factor NMNAT2. Elife 6:e22540
Yang J et al (2015) Pathological axonal death through a MAPK cascade that triggers a local energy deficit. Cell 160:161–176
Yau C et al (2021) Dysregulated metabolism underpins Zika-virus-infection-associated impairment in fetal development. Cell Rep 37:110118
Thaker SK et al (2019) Differential metabolic reprogramming by zika virus promotes cell death in human versus mosquito cells. Cell Metab 29:1206–1216 e1204
Bouzier-Sore AK, Pellerin L (2013) Unraveling the complex metabolic nature of astrocytes. Front Cell Neurosci 7:179
Bonvento G, Bolanos JP (2021) Astrocyte-neuron metabolic cooperation shapes brain activity. Cell Metab 33:1546–1564
Fan Y, He JJ (2016) HIV-1 Tat Induces unfolded protein response and endoplasmic reticulum stress in astrocytes and causes neurotoxicity through glial fibrillary acidic protein (GFAP) activation and aggregation. J Biol Chem 291:22819–22829
Zhou BY, Liu Y, Kim B, Xiao Y, He JJ (2004) Astrocyte activation and dysfunction and neuron death by HIV-1 Tat expression in astrocytes. Mol Cell Neurosci 27:296–305
Natarajaseenivasan K et al (2018) Astrocytic metabolic switch is a novel etiology for cocaine and HIV-1 Tat-mediated neurotoxicity. Cell Death Dis 9:415
Sivalingam K, Cirino TJ, McLaughlin JP, Samikkannu T (2021) HIV-Tat and cocaine impact brain energy metabolism: redox modification and mitochondrial biogenesis influence NRF transcription-mediated neurodegeneration. Mol Neurobiol 58:490–504
Crunfli F, Carregari VC, Veras FP, Silva LS, Nogueira MH, Antunes ASLM, Vendramini PH, Valença AGF, Brandão-Teles C, Zuccoli GDS, Reis-de-Oliveira G, Silva-Costa LC, Saia-Cereda VM, Smith BJ, Codo AC, de Souza GF, Muraro SP, Parise PL, Toledo-Teixeira DA, Santos de Castro ÍM, Melo BM, Almeida GM, Firmino EMS, Paiva IM, Silva BMS, Guimarães RM, Mendes ND, Ludwig RL, Ruiz GP, Knittel TL, Davanzo GG, Gerhardt JA, Rodrigues PB, Forato J, Amorim MR, Brunetti NS, Martini MC, Benatti MN, Batah SS, Siyuan L, João RB, Aventurato ÍK, Rabelo de Brito M, Mendes MJ, da Costa BA, Alvim MKM, da Silva Júnior JR, Damião LL, de Sousa IMP, da Rocha ED, Gonçalves SM, Lopes da Silva LH, Bettini V, Campos BM, Ludwig G, Tavares LA, Pontelli MC, Viana RMM, Martins RB, Vieira AS, Alves-Filho JC, Arruda E, Podolsky-Gondim GG, Santos MV, Neder L, Damasio A, Rehen S, Vinolo MAR, Munhoz CD, Louzada-Junior P, Oliveira RD, Cunha FQ, Nakaya HI, Mauad T, Duarte-Neto AN, Ferraz da Silva LF, Dolhnikoff M, Saldiva PHN, Farias AS, Cendes F, Moraes-Vieira PMM, Fabro AT, Sebollela A, Proença-Modena JL, Yasuda CL, Mori MA, Cunha TM, Martins-de-Souza D (2022) Morphological, cellular, and molecular basis of brain infection in COVID-19 patients. Proc Natl Acad Sci U S A 119(35):e2200960119. https://doi.org/10.1073/pnas.2200960119
L. G. de Oliveira et al., SARS-CoV-2 infection impacts carbon metabolism and depends on glutamine for replication in Syrian hamster astrocytes. 10.1101/2021.10.23.465567 %J bioRxiv, 2021.2010.2023.465567 (2021).
Missale C, Nash SR, Robinson SW, Jaber M, Caron MG (1998) Dopamine receptors: from structure to function. Physiol Rev 78:189–225
Simanjuntak Y, Liang JJ, Lee YL, Lin YL (2017) Japanese encephalitis virus exploits dopamine D2 receptor-phospholipase C to target dopaminergic human neuronal cells. Front Microbiol 8:651
Misra UK et al (2009) A study of motor activity and catecholamine levels in different brain regions following Japanese encephalitis virus infection in rats. Brain Res 1292:136–147
Chauhan PS, Khanna VK, Kalita J, Misra UK (2017) Japanese encephalitis virus infection results in transient dysfunction of memory learning and cholinesterase inhibition. Mol Neurobiol 54:4705–4715
Chauhan PS, Misra UK, Kalita J, Chandravanshi LP, Khanna VK (2016) Memory and learning seems to be related to cholinergic dysfunction in the JE rat model. Physiol Behav 156:148–155
Ford TC, Nibbs R, Crewther DP (2017) Increased glutamate/GABA+ ratio in a shared autistic and schizotypal trait phenotype termed social disorganisation. Neuroimage Clin 16:125–131
Bonansco C, Fuenzalida M (2016) plasticity of hippocampal excitatory-inhibitory balance: missing the synaptic control in the epileptic brain. Neural Plast 2016:8607038
Bak LK, Schousboe A, Waagepetersen HS (2006) The glutamate/GABA-glutamine cycle: aspects of transport, neurotransmitter homeostasis and ammonia transfer. J Neurochem 98:641–653
Sailasuta N, Shriner K, Ross B (2009) Evidence of reduced glutamate in the frontal lobe of HIV-seropositive patients. NMR Biomed 22:326–331
Young AC et al (2014) Cerebral metabolite changes prior to and after antiretroviral therapy in primary HIV infection. Neurology 83:1592–1600
Ernst T, Jiang CS, Nakama H, Buchthal S, Chang L (2010) Lower brain glutamate is associated with cognitive deficits in HIV patients: a new mechanism for HIV-associated neurocognitive disorder. J Magn Reson Imaging 32:1045–1053
Ferrarese C et al (2001) Increased glutamate in CSF and plasma of patients with HIV dementia. Neurology 57:671–675
Gorska AM, Eugenin EA (2020) the glutamate system as a crucial regulator of CNS toxicity and survival of HIV reservoirs. Front Cell Infect Microbiol 10:261
Eugenin EA et al (2007) HIV-tat induces formation of an LRP-PSD-95- NMDAR-nNOS complex that promotes apoptosis in neurons and astrocytes. Proc Natl Acad Sci U S A 104:3438–3443
Prendergast MA et al (2002) Neurotoxic effects of the human immunodeficiency virus type-1 transcription factor Tat require function of a polyamine sensitive-site on the N-methyl-D-aspartate receptor. Brain Res 954:300–307
Kim HJ, Martemyanov KA, Thayer SA (2008) Human immunodeficiency virus protein Tat induces synapse loss via a reversible process that is distinct from cell death. J Neurosci 28:12604–12613
Huang Y et al (2011) Glutaminase dysregulation in HIV-1-infected human microglia mediates neurotoxicity: relevant to HIV-1-associated neurocognitive disorders. J Neurosci 31:15195–15204
Vazquez-Santiago FJ, Noel RJ Jr, Porter JT, Rivera-Amill V (2014) Glutamate metabolism and HIV-associated neurocognitive disorders. J Neurovirol 20:315–331
Chauhan PS, Misra UK, Kalita J (2017) A study of glutamate levels, NR1, NR2A, NR2B receptors and oxidative stress in rat model of Japanese encephalitis. Physiol Behav 171:256–267
Chen CJ et al (2012) Glutamate released by Japanese encephalitis virus-infected microglia involves TNF-alpha signaling and contributes to neuronal death. Glia 60:487–501
Selkoe DJ, Hardy J (2016) The amyloid hypothesis of Alzheimer's disease at 25 years. EMBO Mol Med 8:595–608
Choy RW, Cheng Z, Schekman R (2012) Amyloid precursor protein (APP) traffics from the cell surface via endosomes for amyloid beta (Abeta) production in the trans-Golgi network. Proc Natl Acad Sci U S A 109:E2077–E2082
Multhaup G, Huber O, Buee L, Galas MC (2015) amyloid precursor protein (APP) metabolites APP intracellular fragment (AICD), Abeta42, and Tau in nuclear roles. J Biol Chem 290:23515–23522
O'Brien RJ, Wong PC (2011) Amyloid precursor protein processing and Alzheimer's disease. Annu Rev Neurosci 34:185–204
Querfurth HW, LaFerla FM (2010) Alzheimer's disease. N Engl J Med 362:329–344
Reddy PH (2013) Amyloid beta-induced glycogen synthase kinase 3beta phosphorylated VDAC1 in Alzheimer's disease: implications for synaptic dysfunction and neuronal damage. Biochim Biophys Acta 1832:1913–1921
Lewis J et al (2001) Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science 293:1487–1491
Cummings JL (2004) Alzheimer's disease. N Engl J Med 351:56–67
Laval K, Enquist LW (2021) The potential role of herpes simplex virus type 1 and neuroinflammation in the pathogenesis of Alzheimer's disease. Front Neurol 12:658695
Sait A, Angeli C, Doig AJ, Day PJR (2021) viral involvement in Alzheimer's disease. ACS Chem Neurosci 12:1049–1060
Borrajo A, Spuch C, Penedo MA, Olivares JM, Agis-Balboa RC (2021) Important role of microglia in HIV-1 associated neurocognitive disorders and the molecular pathways implicated in its pathogenesis. Ann Med 53:43–69
Harris SA, Harris EA (2018) Molecular mechanisms for herpes simplex virus type 1 pathogenesis in Alzheimer's disease. Front Aging Neurosci 10:48
Canet G et al (2018) HIV Neuroinfection and Alzheimer's disease: similarities and potential links? Front Cell Neurosci 12:307
Wozniak MA, Mee AP, Itzhaki RF (2009) Herpes simplex virus type 1 DNA is located within Alzheimer's disease amyloid plaques. J Pathol 217:131–138
Deatly AM, Haase AT, Fewster PH, Lewis E, Ball MJ (1990) human herpes virus infections and Alzheimer's disease. Neuropathol Appl Neurobiol 16:213–223
Lin WR, Wozniak MA, Cooper RJ, Wilcock GK, Itzhaki RF (2002) Herpesviruses in brain and Alzheimer's disease. J Pathol 197:395–402
Wozniak MA, Shipley SJ, Combrinck M, Wilcock GK, Itzhaki RF (2005) Productive herpes simplex virus in brain of elderly normal subjects and Alzheimer's disease patients. J Med Virol 75:300–306
Readhead B et al (2018) Multiscale analysis of independent Alzheimer's cohorts finds disruption of molecular, genetic, and clinical networks by human herpesvirus. Neuron 99:64–82 e67
Ball MJ (1982) Limbic predilection in Alzheimer dementia: is reactivated herpesvirus involved? Can J Neurol Sci 9:303–306
De Chiara G et al (2010) APP processing induced by herpes simplex virus type 1 (HSV-1) yields several APP fragments in human and rat neuronal cells. PLoS One 5:e13989
Cairns DM et al (2020) A 3D human brain-like tissue model of herpes-induced Alzheimer's disease. Sci Adv 6:eaay8828
Santana S, Recuero M, Bullido MJ, Valdivieso F, Aldudo J (2012) Herpes simplex virus type I induces the accumulation of intracellular beta-amyloid in autophagic compartments and the inhibition of the non-amyloidogenic pathway in human neuroblastoma cells. Neurobiol Aging 33(430):e419–e433
Wozniak MA, Itzhaki RF, Shipley SJ, Dobson CB (2007) Herpes simplex virus infection causes cellular beta-amyloid accumulation and secretase upregulation. Neurosci Lett 429:95–100
Santana S, Sastre I, Recuero M, Bullido MJ, Aldudo J (2013) Oxidative stress enhances neurodegeneration markers induced by herpes simplex virus type 1 infection in human neuroblastoma cells. PLoS One 8:e75842
Piacentini R et al (2011) HSV-1 promotes Ca2+ -mediated APP phosphorylation and Abeta accumulation in rat cortical neurons. Neurobiol Aging 32(2323):e2313–e2326
De Chiara G et al (2019) Recurrent herpes simplex virus-1 infection induces hallmarks of neurodegeneration and cognitive deficits in mice. PLoS Pathog 15:e1007617
Civitelli L et al (2015) Herpes simplex virus type 1 infection in neurons leads to production and nuclear localization of APP intracellular domain (AICD): implications for Alzheimer's disease pathogenesis. J Neurovirol 21:480–490
Piacentini R et al (2015) Herpes simplex virus type-1 infection induces synaptic dysfunction in cultured cortical neurons via GSK-3 activation and intraneuronal amyloid-beta protein accumulation. Sci Rep 5:15444
Martin C et al (2014) Inflammatory and neurodegeneration markers during asymptomatic HSV-1 reactivation. J Alzheimers Dis 39:849–859
Lerchundi R et al (2011) Tau cleavage at D421 by caspase-3 is induced in neurons and astrocytes infected with herpes simplex virus type 1. J Alzheimers Dis 23:513–520
Alvarez G, Aldudo J, Alonso M, Santana S, Valdivieso F (2012) Herpes simplex virus type 1 induces nuclear accumulation of hyperphosphorylated tau in neuronal cells. J Neurosci Res 90:1020–1029
Wozniak MA, Frost AL, Itzhaki RF (2009) Alzheimer's disease-specific tau phosphorylation is induced by herpes simplex virus type 1. J Alzheimers Dis 16:341–350
Liu X, Cohen JI (2015) The role of PI3K/Akt in human herpesvirus infection: From the bench to the bedside. Virology 479-480:568–577
Zheng K et al (2014) Epidermal growth factor receptor-PI3K signaling controls cofilin activity to facilitate herpes simplex virus 1 entry into neuronal cells. mBio 5:e00958–e00913
Ploia C et al (2010) Role of glycogen synthase kinase-3beta in APP hyperphosphorylation induced by NMDA stimulation in cortical neurons. Pharmaceuticals (Basel) 3:42–58
Medina M, Garrido JJ, Wandosell FG (2011) Modulation of GSK-3 as a therapeutic strategy on Tau pathologies. Front Mol Neurosci 4:24
Kristen H et al (2015) Herpes simplex virus type 2 infection induces AD-like neurodegeneration markers in human neuroblastoma cells. Neurobiol Aging 36:2737–2747
Cairns DM, Itzhaki RF, Kaplan DL (2022) potential involvement of varicella zoster virus in Alzheimer's disease via reactivation of quiescent herpes simplex virus type 1. J Alzheimers Dis 88:1189–1200
Gershon AA et al (2015) Varicella zoster virus infection. Nat Rev Dis Primers 1:15016
Green DA et al (2005) Brain deposition of beta-amyloid is a common pathologic feature in HIV positive patients. AIDS 19:407–411
Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE (2006) Accelerated Tau deposition in the brains of individuals infected with human immunodeficiency virus-1 before and after the advent of highly active anti-retroviral therapy. Acta Neuropathol 111:529–538
Brew BJ, Pemberton L, Blennow K, Wallin A, Hagberg L (2005) CSF amyloid beta42 and tau levels correlate with AIDS dementia complex. Neurology 65:1490–1492
Giunta B et al (2009) HIV-1 Tat contributes to Alzheimer's disease-like pathology in PSAPP mice. Int J Clin Exp Pathol 2:433–443
Kim BO et al (2003) Neuropathologies in transgenic mice expressing human immunodeficiency virus type 1 Tat protein under the regulation of the astrocyte-specific glial fibrillary acidic protein promoter and doxycycline. Am J Pathol 162:1693–1707
Kim J, Yoon JH, Kim YS (2013) HIV-1 Tat interacts with and regulates the localization and processing of amyloid precursor protein. PLoS One 8:e77972
Soliman ML, Geiger JD, Chen X (2017) Caffeine blocks HIV-1 Tat-induced amyloid beta production and Tau phosphorylation. J Neuroimmune Pharmacol 12:163–170
Naghavi MH (2018) "APP"reciating the complexity of HIV-induced neurodegenerative diseases. PLoS Pathog 14:e1007309
Chen X, Hui L, Geiger NH, Haughey NJ, Geiger JD (2013) Endolysosome involvement in HIV-1 transactivator protein-induced neuronal amyloid beta production. Neurobiol Aging 34:2370–2378
Hategan A, Masliah E, Nath A (2019) HIV and Alzheimer's disease: complex interactions of HIV-Tat with amyloid beta peptide and Tau protein. J Neurovirol 25:648–660
Forman MS, Trojanowski JQ, Lee VM (2004) Neurodegenerative diseases: a decade of discoveries paves the way for therapeutic breakthroughs. Nat Med 10:1055–1063
Goedert M (2001) Alpha-synuclein and neurodegenerative diseases. Nat Rev Neurosci 2:492–501
Sejvar JJ et al (2003) Neurologic manifestations and outcome of West Nile virus infection. JAMA 290:511–515
Hsu TW et al (2022) Dengue virus infection and risk of Parkinson's disease: a nationwide longitudinal study. J Parkinsons Dis 12:679–687
Wang H et al (2020) Bacterial, viral, and fungal infection-related risk of Parkinson's disease: meta-analysis of cohort and case-control studies. Brain Behav 10:e01549
Jang H, Boltz DA, Webster RG, Smeyne RJ (2009) Viral parkinsonism. Biochim Biophys Acta 1792:714–721
Dehner LF, Spitz M, Pereira JS (2016) Parkinsonism in HIV infected patients during antiretroviral therapy - data from a Brazilian tertiary hospital. Braz J Infect Dis 20:499–501
Merello M, Bhatia KP, Obeso JA (2021) SARS-CoV-2 and the risk of Parkinson's disease: facts and fantasy. Lancet Neurol 20:94–95
Beatman EL et al (2015) Alpha-synuclein expression restricts RNA viral infections in the brain. J Virol 90:2767–2782
Khanlou N et al (2009) Increased frequency of alpha-synuclein in the substantia nigra in human immunodeficiency virus infection. J Neurovirol 15:131–138
Santerre M et al (2021) HIV-1 Vpr protein impairs lysosome clearance causing SNCA/alpha-synuclein accumulation in neurons. Autophagy 17:1768–1782
Semerdzhiev SA, Fakhree MAA, Segers-Nolten I, Blum C, Claessens M (2022) Interactions between SARS-CoV-2 N-Protein and alpha-Synuclein accelerate amyloid formation. ACS Chem Neurosci 13:143–150
Wu Z, Zhang X, Huang Z, Ma K (2022) SARS-CoV-2 proteins interact with alpha synuclein and induce Lewy body-like pathology in vitro. Int J Mol Sci 23:3394
Swarup V et al (2011) Pathological hallmarks of amyotrophic lateral sclerosis/frontotemporal lobar degeneration in transgenic mice produced with TDP-43 genomic fragments. Brain 134:2610–2626
Saberi S, Stauffer JE, Schulte DJ, Ravits J (2015) Neuropathology of amyotrophic lateral sclerosis and its variants. Neurol Clin 33:855–876
Rahic Z, Buratti E, Cappelli S (2023) Reviewing the potential links between viral infections and TDP-43 proteinopathies. Int J Mol Sci 24:1581
Constant O, Barthelemy J, Nagy A, Salinas S, Simonin Y (2022) West Nile virus neuroinfection in humans: peripheral biomarkers of neuroinflammation and neuronal damage. Viruses 14:756
Laudanski K et al (2021) Dynamic changes in central and peripheral neuro-injury vs. neuroprotective serum markers in COVID-19 are modulated by different types of anti-viral treatments but do not affect the incidence of late and early strokes. Biomedicines 9:1791
Paidas MJ, Cosio DS, Ali S, Kenyon NS, Jayakumar AR (2022) Long-term sequelae of COVID-19 in experimental mice. Mol Neurobiol 59:5970–5986
Rathore A et al (2020) CRISPR-based gene knockout screens reveal deubiquitinases involved in HIV-1 latency in two Jurkat cell models. Sci Rep 10:5350
Cabrera JR, Rodriguez-Izquierdo I, Jimenez JL, Munoz-Fernandez MA (2020) Analysis of ALS-related proteins during herpes simplex virus-2 latent infection. J Neuroinflammation 17:371
Aguzzi A, Sigurdson C, Heikenwaelder M (2008) Molecular mechanisms of prion pathogenesis. Annu Rev Pathol 3:11–40
Hainfellner JA et al (1998) Coexistence of Alzheimer-type neuropathology in Creutzfeldt-Jakob disease. Acta Neuropathol 96:116–122
Prusiner SB (1998) Prions. Proc Natl Acad Sci U S A 95:13363–13383
Voigtlander T et al (2001) Marked increase of neuronal prion protein immunoreactivity in Alzheimer's disease and human prion diseases. Acta Neuropathol 101:417–423
Zafar S et al (2017) Prion protein interactome: identifying novel targets in slowly and rapidly progressive forms of Alzheimer's disease. J Alzheimers Dis 59:265–275
Lauren J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM (2009) Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature 457:1128–1132
Crestini A et al (2022) Prions and neurodegenerative diseases: a focus on Alzheimer's disease. J Alzheimers Dis 85:503–518
Roberson ED et al (2011) Amyloid-beta/Fyn-induced synaptic, network, and cognitive impairments depend on tau levels in multiple mouse models of Alzheimer's disease. J Neurosci 31:700–711
Kong C et al (2019) Binding between prion protein and abeta oligomers contributes to the pathogenesis of Alzheimer's disease. Virol Sin 34:475–488
Zhou J, Liu B (2013) Alzheimer's disease and prion protein. Intractable Rare Dis Res 2:35–44
Brody AH, Strittmatter SM (2018) synaptotoxic signaling by amyloid beta oligomers in Alzheimer's disease through prion protein and mGluR5. Adv Pharmacol 82:293–323
Lathe R, Darlix JL (2020) Prion protein PrP nucleic acid binding and mobilization implicates retroelements as the replicative component of transmissible spongiform encephalopathy. Arch Virol 165:535–556
Hara H et al (2021) Neurotropic influenza A virus infection causes prion protein misfolding into infectious prions in neuroblastoma cells. Sci Rep 11:10109
Roberts TK et al (2010) PrPC, the cellular isoform of the human prion protein, is a novel biomarker of HIV-associated neurocognitive impairment and mediates neuroinflammation. Am J Pathol 177:1848–1860
Acknowledgements
We would like to thank Nattaporn Pakpian for drawing the some of the graphics.
Funding
This research project is supported by the Thailand Research Fund (RSA6280035) and Specific League Funds from Mahidol University for PW.
Author information
Authors and Affiliations
Contributions
The contribution of each author was as follows: PG suggested and supervised the idea. PW worked on the concept and outline of the review. PW executed the idea and wrote together with TC. TC was responsible for the design and drawing of the main artwork. PW and PG wrote and finalized the final manuscript. All authors have reviewed and agreed to the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wongchitrat, P., Chanmee, T. & Govitrapong, P. Molecular Mechanisms Associated with Neurodegeneration of Neurotropic Viral Infection. Mol Neurobiol 61, 2881–2903 (2024). https://doi.org/10.1007/s12035-023-03761-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03761-6