Abstract
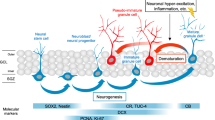
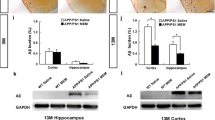
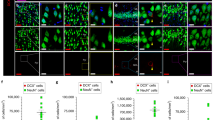
A delicate balance between quiescence and division of the radial glia-like stem cells (RGLs) ensures continuation of adult hippocampal neurogenesis (AHN) over the lifespan. Transient or persistent perturbations of this balance due to a brain pathology, drug administration, or therapy can lead to unfavorable long-term outcomes such as premature depletion of the RGLs, decreased AHN, and cognitive deficit. Memantine, a drug used for alleviating the symptoms of Alzheimer’s disease, and electroconvulsive seizure (ECS), a procedure used for treating drug-resistant major depression or bipolar disorder, are known strong AHN inducers; they were earlier demonstrated to increase numbers of dividing RGLs. Here, we demonstrated that 1-month stimulation of quiescent RGLs by either memantine or ECS leads to premature exhaustion of their pool and altered AHN at later stages of life and that aging of the brain modulates the ability of the quiescent RGLs to be recruited into the cell cycle by these AHN inducers. Our findings support the aging-related divergence of functional features of quiescent RGLs and have a number of implications for the practical assessment of drugs and treatments with respect to their action on quiescent RGLs at different stages of life in animal preclinical studies.





Similar content being viewed by others
Data Availability
All quantitative data are contained within the article. Original microscopy image data sets reported in this paper will be shared by the corresponding author, Dr. Oleg Podgorny (olegpodgorny@inbox.ru), upon request.
References
Altman J (1962) Are new neurons formed in the brains of adult mammals? Science 135:1127–1128. https://doi.org/10.1126/science.135.3509.1127
Cameron HA, Woolley CS, McEwen BS, Gould E (1993) Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience 56:337–344. https://doi.org/10.1016/0306-4522(93)90335-D
Gould E, Reeves AJ, Fallah M, et al (1999) Hippocampal neurogenesis in adult Old World primates. Proc Natl Acad Sci USA 96:5263–5267. https://doi.org/10.1073/pnas.96.9.5263
Kuhn HG, Dickinson-Anson H, Gage FH (1996) Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci 16:2027–2033
van Praag H, Schinder AF, Christie BR et al (2002) Functional neurogenesis in the adult hippocampus. Nature 415:1030–1034. https://doi.org/10.1038/4151030a
Anacker C, Luna VM, Stevens GS et al (2018) Hippocampal neurogenesis confers stress resilience by inhibiting the ventral dentate gyrus. Nature 559:98–102. https://doi.org/10.1038/s41586-018-0262-4
Berdugo-Vega G, Arias-Gil G, López-Fernández A et al (2020) Increasing neurogenesis refines hippocampal activity rejuvenating navigational learning strategies and contextual memory throughout life. Nat Commun 11:135. https://doi.org/10.1038/s41467-019-14026-z
Forte N, Boccella S, Tunisi L et al (2021) Orexin-A and endocannabinoids are involved in obesity-associated alteration of hippocampal neurogenesis, plasticity, and episodic memory in mice. Nat Commun 12:6137. https://doi.org/10.1038/s41467-021-26388-4
Garthe A, Roeder I, Kempermann G (2016) Mice in an enriched environment learn more flexibly because of adult hippocampal neurogenesis. Hippocampus 26:261–271. https://doi.org/10.1002/hipo.22520
Saxe MD, Battaglia F, Wang J-W, et al (2006) Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci USA 103:17501–17506.https://doi.org/10.1073/pnas.0607207103
Tunc-Ozcan E, Peng C-Y, Zhu Y et al (2019) Activating newborn neurons suppresses depression and anxiety-like behaviors. Nat Commun 10:3768. https://doi.org/10.1038/s41467-019-11641-8
Anderson ML, Sisti HM, Curlik DM, Shors TJ (2011) Associative learning increases adult neurogenesis during a critical period. Eur J Neurosci 33:175–181. https://doi.org/10.1111/j.1460-9568.2010.07486.x
Cameron HA, Gould E (1994) Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience 61:203–209. https://doi.org/10.1016/0306-4522(94)90224-0
Gould E, McEwen BS, Tanapat P et al (1997) Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci 17:2492–2498. https://doi.org/10.1523/JNEUROSCI.17-07-02492.1997
Gould E, Beylin A, Tanapat P et al (1999) Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci 2:260–265. https://doi.org/10.1038/6365
van Praag H, Christie BR, Sejnowski TJ, Gage FH (1999) Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci USA 96:13427–13431.https://doi.org/10.1073/pnas.96.23.13427
Toda T, Parylak SL, Linker SB, Gage FH (2019) The role of adult hippocampal neurogenesis in brain health and disease. Mol Psychiatry 24:67–87. https://doi.org/10.1038/s41380-018-0036-2
Terreros-Roncal J, Moreno-Jiménez EP, Flor-García M et al (2021) Impact of neurodegenerative diseases on human adult hippocampal neurogenesis. Science 374:1106–1113. https://doi.org/10.1126/science.abl5163
Moreno-Jiménez EP, Terreros-Roncal J, Flor-García M et al (2021) Evidences for adult hippocampal neurogenesis in humans. J Neurosci 41:2541–2553. https://doi.org/10.1523/JNEUROSCI.0675-20.2020
Sorrells SF, Paredes MF, Zhang Z et al (2021) Positive controls in adults and children support that very few, if any, new neurons are born in the adult human hippocampus. J Neurosci 41:2554–2565. https://doi.org/10.1523/JNEUROSCI.0676-20.2020
Bonaguidi MA, Wheeler MA, Shapiro JS et al (2011) In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell 145:1142–1155. https://doi.org/10.1016/j.cell.2011.05.024
Encinas JM, Michurina TV, Peunova N et al (2011) Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell 8:566–579. https://doi.org/10.1016/j.stem.2011.03.010
Mignone JL, Kukekov V, Chiang A-S et al (2004) Neural stem and progenitor cells in nestin-GFP transgenic mice. J Comp Neurol 469:311–324. https://doi.org/10.1002/cne.10964
Seri B, Garcı́a-Verdugo JM, McEwen BS, Alvarez-Buylla A (2001) Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci 21:7153–7160. https://doi.org/10.1523/JNEUROSCI.21-18-07153.2001
Andersen J, Urbán N, Achimastou A et al (2014) A transcriptional mechanism integrating inputs from extracellular signals to activate hippocampal stem cells. Neuron 83:1085–1097. https://doi.org/10.1016/j.neuron.2014.08.004
Knobloch M, Pilz G-A, Ghesquière B et al (2017) A fatty acid oxidation-dependent metabolic shift regulates adult neural stem cell activity. Cell Rep 20:2144–2155. https://doi.org/10.1016/j.celrep.2017.08.029
Morales AV, Mira H (2019) Adult neural stem cells: born to last. Front Cell Dev Biol 7:96. https://doi.org/10.3389/fcell.2019.00096
Urbán N, van den Berg DLC, Forget A et al (2016) Return to quiescence of mouse neural stem cells by degradation of a proactivation protein. Science 353:292–295. https://doi.org/10.1126/science.aaf4802
Zhou Y, Bond AM, Shade JE et al (2018) Autocrine Mfge8 signaling prevents developmental exhaustion of the adult neural stem cell pool. Cell Stem Cell 23:444-452.e4. https://doi.org/10.1016/j.stem.2018.08.005
Sierra A, Encinas JM, Deudero JJP et al (2010) Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell 7:483–495. https://doi.org/10.1016/j.stem.2010.08.014
Lazutkin A, Podgorny O, Enikolopov G (2019) Modes of division and differentiation of neural stem cells. Behav Brain Res 374:112118. https://doi.org/10.1016/j.bbr.2019.112118
Martín-Suárez S, Valero J, Muro-García T, Encinas JM (2019) Phenotypical and functional heterogeneity of neural stem cells in the aged hippocampus. Aging Cell 18:e12958. https://doi.org/10.1111/acel.12958
Pilz G-A, Bottes S, Betizeau M et al (2018) Live imaging of neurogenesis in the adult mouse hippocampus. Science 359:658–662. https://doi.org/10.1126/science.aao5056
Jang M-H, Bonaguidi MA, Kitabatake Y et al (2013) Secreted frizzled-related protein 3 regulates activity-dependent adult hippocampal neurogenesis. Cell Stem Cell 12:215–223. https://doi.org/10.1016/j.stem.2012.11.021
Kang W, Hebert JM (2015) FGF signaling is necessary for neurogenesis in young mice and sufficient to reverse its decline in old mice. J Neurosci 35:10217–10223. https://doi.org/10.1523/JNEUROSCI.1469-15.2015
Bao H, Asrican B, Li W et al (2017) Long-range GABAergic inputs regulate neural stem cell quiescence and control adult hippocampal neurogenesis. Cell Stem Cell 21:604-617.e5. https://doi.org/10.1016/j.stem.2017.10.003
Yeh C-Y, Asrican B, Moss J et al (2018) Mossy cells control adult neural stem cell quiescence and maintenance through a dynamic balance between direct and indirect pathways. Neuron 99:493-510.e4. https://doi.org/10.1016/j.neuron.2018.07.010
Groves N, O’Keeffe I, Lee W et al (2019) Blockade of TrkB but not p75 NTR activates a subpopulation of quiescent neural precursor cells and enhances neurogenesis in the adult mouse hippocampus. Develop Neurobiol 79:868–879. https://doi.org/10.1002/dneu.22729
Kalamakis G, Brüne D, Ravichandran S et al (2019) Quiescence modulates stem cell maintenance and regenerative capacity in the aging brain. Cell 176:1407-1419.e14. https://doi.org/10.1016/j.cell.2019.01.040
Adusumilli VS, Walker TL, Overall RW et al (2021) ROS dynamics delineate functional states of hippocampal neural stem cells and link to their activity-dependent exit from quiescence. Cell Stem Cell 28:300-314.e6. https://doi.org/10.1016/j.stem.2020.10.019
Petrelli F, Scandella V, Montessuit S et al (2023) Mitochondrial pyruvate metabolism regulates the activation of quiescent adult neural stem cells. Sci Adv 9:eadd5220. https://doi.org/10.1126/sciadv.add5220
Bottes S, Jaeger BN, Pilz G-A et al (2021) Long-term self-renewing stem cells in the adult mouse hippocampus identified by intravital imaging. Nat Neurosci 24:225–233. https://doi.org/10.1038/s41593-020-00759-4
Harris L, Rigo P, Stiehl T et al (2021) Coordinated changes in cellular behavior ensure the lifelong maintenance of the hippocampal stem cell population. Cell Stem Cell 28:863-876.e6. https://doi.org/10.1016/j.stem.2021.01.003
Ibrayeva A, Bay M, Pu E et al (2021) Early stem cell aging in the mature brain. Cell Stem Cell 28:955-966.e7. https://doi.org/10.1016/j.stem.2021.03.018
Segi-Nishida E, Warner-Schmidt JL, Duman RS (2008) Electroconvulsive seizure and VEGF increase the proliferation of neural stem-like cells in rat hippocampus. Proc Natl Acad Sci U S A 105:11352–11357. https://doi.org/10.1073/pnas.0710858105
Namba T, Maekawa M, Yuasa S et al (2009) The Alzheimer’s disease drug memantine increases the number of radial glia-like progenitor cells in adult hippocampus. Glia 57:1082–1090. https://doi.org/10.1002/glia.20831
Itaman S, Enikolopov G, Podgorny OV (2022) Detection of de novo dividing stem cells in situ through double nucleotide analogue labeling. Cells 11:4001. https://doi.org/10.3390/cells11244001
Mandyam CD, Harburg GC, Eisch AJ (2007) Determination of key aspects of precursor cell proliferation, cell cycle length and kinetics in the adult mouse subgranular zone. Neuroscience 146:108–122. https://doi.org/10.1016/j.neuroscience.2006.12.064
Encinas JM, Enikolopov G (2008) Identifying and quantitating neural stem and progenitor cells in the adult brain. In: Methods in Cell Biology. Elsevier, pp 243–272
Maltsev DI, Mellanson KA, Belousov VV et al (2022) The bioavailability time of commonly used thymidine analogues after intraperitoneal delivery in mice: labeling kinetics in vivo and clearance from blood serum. Histochem Cell Biol 157:239–250. https://doi.org/10.1007/s00418-021-02048-y
Podgorny O, Peunova N, Park J-H, Enikolopov G (2018) Triple S-phase labeling of dividing stem cells. Stem Cell Reports 10:615–626. https://doi.org/10.1016/j.stemcr.2017.12.020
Maekawa M, Namba T, Suzuki E et al (2009) NMDA receptor antagonist memantine promotes cell proliferation and production of mature granule neurons in the adult hippocampus. Neurosci Res 63:259–266. https://doi.org/10.1016/j.neures.2008.12.006
Kronenberg G, Reuter K, Steiner B et al (2003) Subpopulations of proliferating cells of the adult hippocampus respond differently to physiologic neurogenic stimuli. J Comp Neurol 467:455–463. https://doi.org/10.1002/cne.10945
Seri B, García-Verdugo JM, Collado-Morente L et al (2004) Cell types, lineage, and architecture of the germinal zone in the adult dentate gyrus: organization of the Vertebrate Dentate Gyrus. J Comp Neurol 478:359–378. https://doi.org/10.1002/cne.20288
Moss J, Gebara E, Bushong EA, et al (2016) Fine processes of Nestin-GFP–positive radial glia-like stem cells in the adult dentate gyrus ensheathe local synapses and vasculature. Proc Natl AcadSci USA 113. https://doi.org/10.1073/pnas.1514652113
Brandt MD, Hübner M, Storch A (2012) Brief report: adult hippocampal precursor cells shorten S-phase and total cell cycle length during neuronal differentiation. Stem Cells 30:2843–2847. https://doi.org/10.1002/stem.1244
Fischer TJ, Walker TL, Overall RW et al (2014) Acute effects of wheel running on adult hippocampal precursor cells in mice are not caused by changes in cell cycle length or S phase length. Front Neurosci 8:314. https://doi.org/10.3389/fnins.2014.00314
Solius GM, Maltsev DI, Belousov VV, Podgorny OV (2021) Recent advances in nucleotide analogue-based techniques for tracking dividing stem cells: an overview. J Biol Chem 297:101345. https://doi.org/10.1016/j.jbc.2021.101345
Sun D, Chen J, Bao X et al (2015) Protection of radial glial-like cells in the hippocampus of APP/PS1 mice: a novel mechanism of memantine in the treatment of Alzheimer’s disease. Mol Neurobiol 52:464–477. https://doi.org/10.1007/s12035-014-8875-6
Sierra A, Martín-Suárez S, Valcárcel-Martín R et al (2015) Neuronal hyperactivity accelerates depletion of neural stem cells and impairs hippocampal neurogenesis. Cell Stem Cell 16:488–503. https://doi.org/10.1016/j.stem.2015.04.003
Cameron HA, McKay RD (1999) Restoring production of hippocampal neurons in old age. Nat Neurosci 2:894–897. https://doi.org/10.1038/13197
Montaron MF, Drapeau E, Dupret D et al (2006) Lifelong corticosterone level determines age-related decline in neurogenesis and memory. Neurobiol Aging 27:645–654. https://doi.org/10.1016/j.neurobiolaging.2005.02.014
Villeda SA, Luo J, Mosher KI et al (2011) The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 477:90–94. https://doi.org/10.1038/nature10357
Fatt MP, Tran LM, Vetere G et al (2022) Restoration of hippocampal neural precursor function by ablation of senescent cells in the aging stem cell niche. Stem Cell Reports 17:259–275. https://doi.org/10.1016/j.stemcr.2021.12.010
Cole JD, Sarabia Del Castillo J, Gut G et al (2022) Characterization of the neurogenic niche in the aging dentate gyrus using iterative immunofluorescence imaging. eLife 11:e68000. https://doi.org/10.7554/eLife.68000
Martín-Suárez S, Encinas JM (2021) The future belongs to those who prepare for it today. Cell Stem Cell 28:783–785. https://doi.org/10.1016/j.stem.2021.04.014
Marvanová M, Lakso M, Pirhonen J et al (2001) The neuroprotective agent memantine induces brain-derived neurotrophic factor and trkB receptor expression in rat brain. Mol Cell Neurosci 18:247–258. https://doi.org/10.1006/mcne.2001.1027
Lee J, Duan W, Mattson MP (2002) Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice: BDNF, dietary restriction and neurogenesis. J Neurochem 82:1367–1375. https://doi.org/10.1046/j.1471-4159.2002.01085.x
Li Y, Luikart BW, Birnbaum S et al (2008) TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron 59:399–412. https://doi.org/10.1016/j.neuron.2008.06.023
Tapley P, Lamballe F, Barbacid M (1992) K252a is a selective inhibitor of the tyrosine protein kinase activity of the trk family of oncogenes and neurotrophin receptors. Oncogene 7:371–381
Pinnock SB, Blake AM, Platt NJ, Herbert J (2010) The roles of BDNF, pCREB and Wnt3a in the latent period preceding activation of progenitor cell mitosis in the adult dentate gyrus by fluoxetine. PLoS One 5:e13652. https://doi.org/10.1371/journal.pone.0013652
Warner-Schmidt JL, Duman RS (2007) VEGF is an essential mediator of the neurogenic and behavioral actions of antidepressants. Proc Natl Acad Sci U S A 104:4647–4652.https://doi.org/10.1073/pnas.0610282104
Fong TA, Shawver LK, Sun L et al (1999) SU5416 is a potent and selective inhibitor of the vascular endothelial growth factor receptor (Flk-1/KDR) that inhibits tyrosine kinase catalysis, tumor vascularization, and growth of multiple tumor types. Cancer Res 59:99–106
Ehm O, Göritz C, Covic M et al (2010) RBPJkappa-dependent signaling is essential for long-term maintenance of neural stem cells in the adult hippocampus. J Neurosci 30:13794–13807. https://doi.org/10.1523/JNEUROSCI.1567-10.2010
Jonckheere J, Deloulme J-C, Dall’Igna G et al (2018) Short- and long-term efficacy of electroconvulsive stimulation in animal models of depression: the essential role of neuronal survival. Brain Stimulation 11:1336–1347. https://doi.org/10.1016/j.brs.2018.08.001
Sairanen M, Lucas G, Ernfors P et al (2005) Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. J Neurosci 25:1089–1094. https://doi.org/10.1523/JNEUROSCI.3741-04.2005
Rossi C, Angelucci A, Costantin L et al (2006) Brain-derived neurotrophic factor (BDNF) is required for the enhancement of hippocampal neurogenesis following environmental enrichment. Eur J Neurosci 24:1850–1856. https://doi.org/10.1111/j.1460-9568.2006.05059.x
Ponti G, Obernier K, Guinto C, et al (2013) Cell cycle and lineage progression of neural progenitors in the ventricular-subventricular zones of adult mice. Proc Natl Acad Sci USA 110:E1045–E1054.https://doi.org/10.1073/pnas.1219563110
Kohlmeier F, Maya-Mendoza A, Jackson DA (2013) EdU induces DNA damage response and cell death in mESC in culture. Chromosome Res 21:87–100. https://doi.org/10.1007/s10577-013-9340-5
Palmer TD, Willhoite AR, Gage FH (2000) Vascular niche for adult hippocampal neurogenesis. J Comp Neurol 425:479–494. https://doi.org/10.1002/1096-9861(20001002)425:4%3c479::aid-cne2%3e3.0.co;2-3
Sharma RB, Darko C, Zheng X et al (2019) DNA Damage does not cause BrdU labeling of mouse or human β-cells. Diabetes 68:975–987. https://doi.org/10.2337/db18-0761
Funding
This study was funded by the Russian Foundation for Basic Research, grant no. 19–29-04016 (to O.V.P.); the Ministry of Science and Higher Education of the Russian Federation, grant no. 075–15-2019–1789 to the Center for Precision Genome Editing and Genetic Technologies for Biomedicine (to O.V.P.); and the state assignment of the Ministry of Science and Higher Education of the Russian Federation for 2021–2023 (V.A.A. and N.V.G.).
Author information
Authors and Affiliations
Contributions
V.V.B., N.V.G., and O.V.P. designed the experiments. D.I.M., V.A.A., M.A.G., and A.D.P. performed and analyzed the experiments. N.V.G., and O.V.P. interpreted the results and wrote the manuscript. V.V.B. edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
All manipulations with animals were conducted according to the European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes (1986, ETS 123) and approved by the Institutional Animal Care and Use Committee of Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry (approval no. 281).
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1. Online Resource 1 A cell of the EdU+BrdU-GFP+GFAP+process+ phenotype (a de novo dividing bona fide RGL stem cell). The video illustrates a Z-series of consecutive optical slices obtained using the confocal microscope. The arrow shows the body of an EdU+BrdU- RGL stem cell. The arrowheads show a GFP+GFAP+ apical process of the RGL stem cell (AVI 4530 KB)
Supplementary file2. Online Resource 2 A cell of the EdU+BrdU-GFP+GFAP+process- phenotype (a de novo dividing presumptive RGL stem cell). The video illustrates a Z-series of consecutive optical slices obtained using the confocal microscope. The arrow shows the body of an EdU+BrdU- presumptive RGL stem cell (AVI 1095 KB)
Supplementary file3. Online Resource 3 A cell of the EdU+BrdU-GFP+GFAP-process- phenotype. The video illustrates a Z-series of consecutive optical slices obtained using the confocal microscope. The arrow shows the body of an EdU+BrdU-GFP+GFAP-process- cell (AVI 731 KB)
Supplementary file4. Online Resource 4 Two cells of the EdU+BrdU-GFP-GFAP-process- phenotype. The video illustrates a Z-series of consecutive optical slices obtained using the confocal microscope. The arrows show bodies of two EdU+BrdU-GFP-GFAP-process- cell (AVI 3454 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Maltsev, D.I., Aniol, V.A., Golden, M.A. et al. Aging Modulates the Ability of Quiescent Radial Glia-Like Stem Cells in the Hippocampal Dentate Gyrus to be Recruited into Division by Pro-neurogenic Stimuli. Mol Neurobiol (2023). https://doi.org/10.1007/s12035-023-03746-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12035-023-03746-5