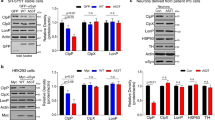
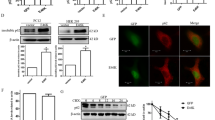
Abstract
Parkinsonism is a clinical syndrome that is caused by Parkinson’s disease (PD) and other neurodegenerative diseases. Here, we report a patient who exhibited progressive parkinsonism, epilepsy, and cognitive impairment and was diagnosed with adult-onset neuronal ceroid lipofuscinoses (ANCLs). The patient carries a mutation (p.Leu116 del) in the DNAJC5 gene that encodes cysteine string protein (CSPα). Since the patient shows typical parkinsonism and loss of dopamine transporter in the striatum, we investigated the effect of wild-type and L116del mutant CSPα on the aggregation of α-synuclein (α-syn) and neurotoxicity in vitro. Overexpression of wild-type CSPα attenuated the phosphorylation, ubiquitination, and aggregation of α-syn induced by α-syn fibrils. Moreover, wild-type CSPα inhibits oxidative stress and cell apoptosis and rescues inefficient SNARE complex formation induced by α-syn fibrils in SH-SY5Y cells. However, these protective effects of CSPα were abolished by the L116del mutation. Collectively, these results indicate that L116 deletion in CSPα promotes α-syn pathology and neurotoxicity. Boosting CSPα may be therapeutically useful for treating synucleinopathies.






Similar content being viewed by others
Data Availability
All data generated or analyzed in this study are available in the published article.
Code Availability
Not applicable.
References
Naseri N, Sharma M, Velinov M (2021) Autosomal dominant neuronal ceroid lipofuscinosis: clinical features and molecular basis. Clin Genet 99:111–118. https://doi.org/10.1111/cge.13829
Greaves J, Lemonidis K, Gorleku OA et al (2012) Palmitoylation-induced aggregation of cysteine-string protein mutants that cause neuronal ceroid lipofuscinosis. J Biol Chem 287:37330–37339. https://doi.org/10.1074/jbc.M112.389098
Nosková L, Stránecký V, Hartmannová H et al (2011) Mutations in DNAJC5, encoding cysteine-string protein alpha, cause autosomal-dominant adult-onset neuronal ceroid lipofuscinosis. Am J Hum Genet 89:241–252. https://doi.org/10.1016/j.ajhg.2011.07.003
Benitez BA, Alvarado D, Cai Y et al (2011) Exome-sequencing confirms DNAJC5 mutations as cause of adult neuronal ceroid-lipofuscinosis. PLoS One 6:e26741. https://doi.org/10.1371/journal.pone.0026741
Benitez BA, Cairns NJ, Schmidt RE et al (2015) Clinically early-stage CSPα mutation carrier exhibits remarkable terminal stage neuronal pathology with minimal evidence of synaptic loss. Acta Neuropathol Commun 3:73. https://doi.org/10.1186/s40478-015-0256-5
Burgoyne RD, Morgan A (2015) Cysteine string protein (CSP) and its role in preventing neurodegeneration. Semin Cell Dev Biol 40:153–159. https://doi.org/10.1016/j.semcdb.2015.03.008
Caló L, Hidari E, Wegrzynowicz M et al (2021) CSPα reduces aggregates and rescues striatal dopamine release in α-synuclein transgenic mice. Brain 144:1661–1669. https://doi.org/10.1093/brain/awab076
Sharma M, Burré J, Südhof TC (2011) CSPα promotes SNARE-complex assembly by chaperoning SNAP-25 during synaptic activity. Nat Cell Biol 13:30–39. https://doi.org/10.1038/ncb2131
Tobaben S, Thakur P, Fernández-Chacón R et al (2001) A trimeric protein complex functions as a synaptic chaperone machine. Neuron 31:987–999. https://doi.org/10.1016/s0896-6273(01)00427-5
Garcia-Reitböck P, Anichtchik O, Bellucci A et al (2010) SNARE protein redistribution and synaptic failure in a transgenic mouse model of Parkinson’s disease. Brain 133:2032–2044. https://doi.org/10.1093/brain/awq132
Levin J, Kurz A, Arzberger T et al (2016) The differential diagnosis and treatment of atypical parkinsonism. Dtsch Arztebl Int 113:61–69. https://doi.org/10.3238/arztebl.2016.0061
Grammatopoulos TN, Outeiro TF, Hyman BT, Standaert DG (2007) Angiotensin II protects against alpha-synuclein toxicity and reduces protein aggregation in vitro. Biochem Biophys Res Commun 363:846–851. https://doi.org/10.1016/j.bbrc.2007.09.043
Chandra S, Gallardo G, Fernández-Chacón R et al (2005) Alpha-synuclein cooperates with CSPalpha in preventing neurodegeneration. Cell 123:383–396. https://doi.org/10.1016/j.cell.2005.09.028
Bozic M, Caus M, Rodrigues-Diez RR et al (2020) Protective role of renal proximal tubular alpha-synuclein in the pathogenesis of kidney fibrosis. Nat Commun 11:1943. https://doi.org/10.1038/s41467-020-15732-9
Cadieux-Dion M, Andermann E, Lachance-Touchette P et al (2013) Recurrent mutations in DNAJC5 cause autosomal dominant Kufs disease. Clin Genet 83:571–575. https://doi.org/10.1111/cge.12020
Burneo JG, Arnold T, Palmer CA et al (2003) Adult-onset neuronal ceroid lipofuscinosis (Kufs disease) with autosomal dominant inheritance in Alabama. Epilepsia 44:841–846. https://doi.org/10.1046/j.1528-1157.2003.39802.x
Huang Q, Zhang Y-F, Li L-J et al (2022) Adult-onset neuronal ceroid lipofuscinosis with a novel DNAJC5 mutation exhibits aberrant protein palmitoylation. Front Aging Neurosci 14:829573. https://doi.org/10.3389/fnagi.2022.829573
Yan M, Xiong M, Dai L et al (2022) Cofilin 1 promotes the pathogenicity and transmission of pathological α-synuclein in mouse models of Parkinson’s disease. NPJ Park Dis 8:1. https://doi.org/10.1038/s41531-021-00272-w
Zhu LN, Qiao HH, Chen L et al (2018) SUMOylation of alpha-synuclein influences on alpha-synuclein aggregation induced by methamphetamine. Front Cell Neurosci 12:1–18. https://doi.org/10.3389/fncel.2018.00262
Musgrove RE, Helwig M, Bae E-J et al (2019) Oxidative stress in vagal neurons promotes parkinsonian pathology and intercellular α-synuclein transfer. J Clin Invest 129:3738–3753. https://doi.org/10.1172/JCI127330
Yang H, Zhang M, Shi J et al (2017) Brain-specific SNAP-25 deletion leads to elevated extracellular glutamate level and schizophrenia-like behavior in mice. Neural Plast 2017:. https://doi.org/10.1155/2017/4526417
Velinov M, Dolzhanskaya N, Gonzalez M et al (2012) Mutations in the gene DNAJC5 cause autosomal dominant kufs disease in a proportion of cases: Study of the parry family and 8 other families. PLoS One 7:1–8. https://doi.org/10.1371/journal.pone.0029729
Sharma M, Burré J, Bronk P et al (2012) CSPα knockout causes neurodegeneration by impairing SNAP-25 function. EMBO J 31:829–841. https://doi.org/10.1038/emboj.2011.467
Gorenberg EL, Chandra SS (2017) The role of co-chaperones in synaptic proteostasis and neurodegenerative disease. Front Neurosci 11:1–16. https://doi.org/10.3389/fnins.2017.00248
Roosen DA, Blauwendraat C, Cookson MR, Lewis PA (2019) DNAJC proteins and pathways to parkinsonism. FEBS J 286:3080–3094. https://doi.org/10.1111/febs.14936
Diez-Ardanuy C, Greaves J, Munro KR et al (2017) A cluster of palmitoylated cysteines are essential for aggregation of cysteine-string protein mutants that cause neuronal ceroid lipofuscinosis. Sci Rep 7:10. https://doi.org/10.1038/s41598-017-00036-8
Zhang Y-Q, Chandra SS (2014) Oligomerization of Cysteine String Protein alpha mutants causing adult neuronal ceroid lipofuscinosis. Biochim Biophys Acta 1842:2136–2146. https://doi.org/10.1016/j.bbadis.2014.07.009
Henderson MX, Wirak GS, Zhang Y-Q et al (2016) Neuronal ceroid lipofuscinosis with DNAJC5/CSPα mutation has PPT1 pathology and exhibit aberrant protein palmitoylation. Acta Neuropathol 131:621–637. https://doi.org/10.1007/s00401-015-1512-2
Bartels T, Choi JG, Selkoe DJ (2011) α-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature 477:107–110. https://doi.org/10.1038/nature10324
Zhang Z, Kang SS, Liu X et al (2017) Asparagine endopeptidase cleaves α-synuclein and mediates pathologic activities in Parkinson’s disease. Nat Struct Mol Biol 24:632–642. https://doi.org/10.1038/nsmb.3433
Jang A, Lee HJ, Suk JE et al (2010) Non-classical exocytosis of α-synuclein is sensitive to folding states and promoted under stress conditions. J Neurochem 113:1263–1274. https://doi.org/10.1111/j.1471-4159.2010.06695.x
Burré J, Sharma M, Tsetsenis T et al (2010) Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science 329:1663–1667. https://doi.org/10.1126/science.1195227
Polymeropoulos MH, Lavedan C, Leroy E et al (1997) Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science 276:2045–2047. https://doi.org/10.1126/science.276.5321.2045
Dai L, Wang J, He M et al (2021) Lovastatin alleviates α-synuclein aggregation and phosphorylation in cellular models of synucleinopathy. Front Mol Neurosci 14:682320. https://doi.org/10.3389/fnmol.2021.682320
Luk KC, Lee VM (2014) Addition of exogenous α-Synuclein Pre-formed fibrils to Primary.pdf. Nat Protoc 9:2135–2146. https://doi.org/10.1038/nprot.2014.143.Addition
Funding
This work was supported by the National Natural Science Foundation of China (No. 82271447 and 82071183), the National Key Research & Development Program of China (2019YFE0115900), and the Innovative Research Groups of Hubei Province (2022CFA026).
Author information
Authors and Affiliations
Contributions
Z.Z., Z.Z., and Y.L. conceived the project. T.G. performed the cellular experiments. J.X., T.X., and M.D. collected the clinical data. H.F. and L.B. performed MRI scanning. L.Z. and J.H. prepared plasmids.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
All animal procedures were performed under the Care and Use of Laboratory Animals guidelines and approved by the Guangdong Academy of Medical Sciences (ER-20200720).
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Tao Guo and Jing Xiong contributed equally to this work.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, T., Xiong, J., Feng, H. et al. L116 Deletion in CSPα Promotes α-Synuclein Aggregation and Neurodegeneration. Mol Neurobiol 61, 15–27 (2024). https://doi.org/10.1007/s12035-023-03552-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03552-z