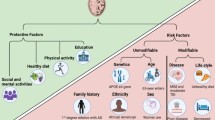
Abstract
Alzheimer’s disease (AD) is the most common type of neurodegenerative disease and its pathogenesis is still unclear. Genetic factors are thought to account for a large proportion of the overall AD phenotypes. ATP-binding cassette transporter A7 (ABCA7) is one of the most important risk gene for AD. Multiple forms of ABCA7 variants significantly increase the risk of AD, such as single-nucleotide polymorphisms, premature termination codon variants, missense variants, variable number tandem repeat, mutations, and alternative splicing. AD patients with ABCA7 variants usually exhibit typical clinical and pathological features of traditional AD with a wide age of onset range. ABCA7 variants can alter ABCA7 protein expression levels and protein structure to affect protein functions such as abnormal lipid metabolism, amyloid precursor protein (APP) processing, and immune cell function. Specifically, ABCA7 deficiency can cause neuronal apoptosis by inducing endoplasmic reticulum stress through the PERK/eIF2α pathway. Second, ABCA7 deficiency can increase Aβ production by upregulating the SREBP2/BACE1 pathway and promoting APP endocytosis. In addition, the ability of microglia to phagocytose and degrade Aβ is destroyed by ABCA7 deficiency, leading to reduced clearance of Aβ. Finally, disturbance of lipid metabolism may also be an important method by which ABCA7 variants influence the incidence rate of AD. In the future, more attention should be given to different ABCA7 variants and ABCA7 targeted therapies for AD.


Similar content being viewed by others
Data Availability
All data are available.
References
Andrews SJ, Fulton-Howard B, Goate A (2020) Interpretation of risk loci from genome-wide association studies of Alzheimer's disease. Lancet Neurol 19(4):326–335. https://doi.org/10.1016/s1474-4422(19)30435-1
Martens YA, Zhao N, Liu CC, Kanekiyo T, Yang AJ, Goate AM, Holtzman DM, Bu G (2022) ApoE cascade hypothesis in the pathogenesis of Alzheimer’s disease and related dementias. Neuron 110(8):1304–1317. https://doi.org/10.1016/j.neuron.2022.03.004
Ridge PG, Hoyt KB, Boehme K, Mukherjee S, Crane PK, Haines JL, Mayeux R, Farrer LA et al (2016) Assessment of the genetic variance of late-onset Alzheimer’s disease. Neurobiol Aging 41:200.e213–200.e220. https://doi.org/10.1016/j.neurobiolaging.2016.02.024
Gatz M, Reynolds CA, Fratiglioni L, Johansson B, Mortimer JA, Berg S, Fiske A, Pedersen NL (2006) Role of genes and environments for explaining Alzheimer disease. Arch Gen Psychiatry 63(2):168–174. https://doi.org/10.1001/archpsyc.63.2.168
Scheltens P, De Strooper B, Kivipelto M, Holstege H, Chételat G, Teunissen CE, Cummings J, van der Flier WM (2021) Alzheimer’s disease. Lancet 397(10284):1577–1590. https://doi.org/10.1016/s0140-6736(20)32205-4
Bellenguez C, Küçükali F, Jansen IE, Kleineidam L, Moreno-Grau S, Amin N, Naj AC, Campos-Martin R et al (2022) New insights into the genetic etiology of Alzheimer’s disease and related dementias. Nat Genet 54(4):412–436. https://doi.org/10.1038/s41588-022-01024-z
Kaminski WE, Orsó E, Diederich W, Klucken J, Drobnik W, Schmitz G (2000) Identification of a novel human sterol-sensitive ATP-binding cassette transporter (ABCA7). Biochem Biophys Res Commun 273(2):532–538. https://doi.org/10.1006/bbrc.2000.2954
Hollingworth P, Harold D, Sims R, Gerrish A, Lambert JC, Carrasquillo MM, Abraham R, Hamshere ML et al (2011) Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer’s disease. Nat Genet 43(5):429–435. https://doi.org/10.1038/ng.803
Stepler KE, Gillyard TR, Reed CB, Avery TM, Davis JS, Robinson RAS (2022) ABCA7, a genetic risk factor associated with Alzheimer’s disease risk in African Americans. J Alzheimer’s Dis 86(1):5–19. https://doi.org/10.3233/jad-215306
Ikeda Y, Abe-Dohmae S, Munehira Y, Aoki R, Kawamoto S, Furuya A, Shitara K, Amachi T et al (2003) Posttranscriptional regulation of human ABCA7 and its function for the apoA-I-dependent lipid release. Biochem Biophys Res Commun 311(2):313–318. https://doi.org/10.1016/j.bbrc.2003.10.002
Dib S, Pahnke J, Gosselet F (2021) Role of ABCA7 in human health and in Alzheimer’s disease. Int J Mol Sci 22(9). https://doi.org/10.3390/ijms22094603
Broccardo C, Osorio J, Luciani MF, Schriml LM, Prades C, Shulenin S, Arnould I, Naudin L et al (2001) Comparative analysis of the promoter structure and genomic organization of the human and mouse ABCA7 gene encoding a novel ABCA transporter. Cytogenet Cell Genet 92(3-4):264–270. https://doi.org/10.1159/000056914
Fu Y, Hsiao JH, Paxinos G, Halliday GM, Kim WS (2016) ABCA7 mediates phagocytic clearance of amyloid-β in the brain. J Alzheimer’s Dis 54(2):569–584. https://doi.org/10.3233/jad-160456
Kim WS, Fitzgerald ML, Kang K, Okuhira K, Bell SA, Manning JJ, Koehn SL, Lu N et al (2005) Abca7 null mice retain normal macrophage phosphatidylcholine and cholesterol efflux activity despite alterations in adipose mass and serum cholesterol levels. J Biol Chem 280(5):3989–3995. https://doi.org/10.1074/jbc.M412602200
Satoh K, Abe-Dohmae S, Yokoyama S, St George-Hyslop P, Fraser PE (2015) ATP-binding cassette transporter A7 (ABCA7) loss of function alters Alzheimer amyloid processing. J Biol Chem 290(40):24152–24165. https://doi.org/10.1074/jbc.M115.655076
Bossaerts L, Hendrickx Van de Craen E, Cacace R, Asselbergh B, Van Broeckhoven C (2022) Rare missense mutations in ABCA7 might increase Alzheimer’s disease risk by plasma membrane exclusion. Acta Neuropathol Commun 10(1):43. https://doi.org/10.1186/s40478-022-01346-3
De Roeck A, Van Broeckhoven C, Sleegers K (2019) The role of ABCA7 in Alzheimer’s disease: evidence from genomics, transcriptomics and methylomics. Acta Neuropathol 138(2):201–220. https://doi.org/10.1007/s00401-019-01994-1
Naj AC, Jun G, Beecham GW, Wang LS, Vardarajan BN, Buros J, Gallins PJ, Buxbaum JD et al (2011) Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer’s disease. Nat Genet 43(5):436–441. https://doi.org/10.1038/ng.801
Reitz C, Jun G, Naj A, Rajbhandary R, Vardarajan BN, Wang LS, Valladares O, Lin CF et al (2013) Variants in the ATP-binding cassette transporter (ABCA7), apolipoprotein E ϵ4,and the risk of late-onset Alzheimer disease in African Americans. JAMA 309(14):1483–1492. https://doi.org/10.1001/jama.2013.2973
Machiela MJ, Chanock SJ (2015) LDlink: a web-based application for exploring population-specific haplotype structure and linking correlated alleles of possible functional variants. Bioinformatics 31(21):3555–3557. https://doi.org/10.1093/bioinformatics/btv402
Jiao B, Xiao X, Yuan Z, Guo L, Liao X, Zhou Y, Zhou L, Wang X et al (2022) Associations of risk genes with onset age and plasma biomarkers of Alzheimer’s disease: a large case-control study in mainland China. Neuropsychopharmacology 47(5):1121–1127. https://doi.org/10.1038/s41386-021-01258-1
Cuyvers E, De Roeck A, Van den Bossche T, Van Cauwenberghe C, Bettens K, Vermeulen S, Mattheijssens M, Peeters K et al (2015) Mutations in ABCA7 in a Belgian cohort of Alzheimer’s disease patients: a targeted resequencing study. Lancet Neurol 14(8):814–822. https://doi.org/10.1016/s1474-4422(15)00133-7
Van den Bossche T, Sleegers K, Cuyvers E, Engelborghs S, Sieben A, De Roeck A, Van Cauwenberghe C, Vermeulen S et al (2016) Phenotypic characteristics of Alzheimer patients carrying an ABCA7 mutation. Neurology 86(23):2126–2133. https://doi.org/10.1212/wnl.0000000000002628
Bossaerts L, Hens E, Hanseeuw B, Vandenberghe R, Cras P, De Deyn PP, Engelborghs S, Van Broeckhoven C (2021) Premature termination codon mutations in ABCA7 contribute to Alzheimer’s disease risk in Belgian patients. Neurobiol Aging 106:307.e301–307.e307. https://doi.org/10.1016/j.neurobiolaging.2021.04.023
De Roeck A, Duchateau L, Van Dongen J, Cacace R, Bjerke M, Van den Bossche T, Cras P, Vandenberghe R et al (2018) An intronic VNTR affects splicing of ABCA7 and increases risk of Alzheimer’s disease. Acta Neuropathol 135(6):827–837. https://doi.org/10.1007/s00401-018-1841-z
Mc Auley MT (2021) DNA methylation in genes associated with the evolution of ageing and disease: a critical review. Ageing Res Rev 72:101488. https://doi.org/10.1016/j.arr.2021.101488
Ilina A, Khavinson V, Linkova N, Petukhov M (2022) Neuroepigenetic mechanisms of action of ultrashort peptides in Alzheimer’s Disease. Int J Mol Sci 23(8). https://doi.org/10.3390/ijms23084259
Poon CH, Tse LSR, Lim LW (2020) DNA methylation in the pathology of Alzheimer’s disease: from gene to cognition. Ann N Y Acad Sci 1475(1):15–33. https://doi.org/10.1111/nyas.14373
De Jager PL, Srivastava G, Lunnon K, Burgess J, Schalkwyk LC, Yu L, Eaton ML, Keenan BT et al (2014) Alzheimer’s disease: early alterations in brain DNA methylation at ANK1, BIN1, RHBDF2 and other loci. Nat Neurosci 17(9):1156–1163. https://doi.org/10.1038/nn.3786
Li QS, Sun Y, Wang T (2020) Epigenome-wide association study of Alzheimer’s disease replicates 22 differentially methylated positions and 30 differentially methylated regions. Clin Epigenetics 12(1):149. https://doi.org/10.1186/s13148-020-00944-z
Chibnik LB, Yu L, Eaton ML, Srivastava G, Schneider JA, Kellis M, Bennett DA, De Jager PL (2015) Alzheimer’s loci: epigenetic associations and interaction with genetic factors. Ann Clin Transl Neurol 2(6):636–647. https://doi.org/10.1002/acn3.201
Yamazaki K, Yoshino Y, Mori T, Yoshida T, Ozaki Y, Sao T, Mori Y, Ochi S et al (2017) Gene expression and methylation analysis of ABCA7 in patients with Alzheimer’s disease. J Alzheimer’s Dis 57(1):171–181. https://doi.org/10.3233/jad-161195
Boden KA, Barber IS, Clement N, Patel T, Guetta-Baranes T, Brookes KJ, Chappell S, Craigon J et al (2017) Methylation profiling RIN3 and MEF2C identifies epigenetic marks associated with sporadic early onset Alzheimer’s disease. J Alzheimer’s Dis 1(1):97–108. https://doi.org/10.3233/adr-170015
Carrasquillo MM, Crook JE, Pedraza O, Thomas CS, Pankratz VS, Allen M, Nguyen T, Malphrus KG et al (2015) Late-onset Alzheimer’s risk variants in memory decline, incident mild cognitive impairment, and Alzheimer’s disease. Neurobiol Aging 36(1):60–67. https://doi.org/10.1016/j.neurobiolaging.2014.07.042
Nettiksimmons J, Tranah G, Evans DS, Yokoyama JS, Yaffe K (2016) Gene-based aggregate SNP associations between candidate AD genes and cognitive decline. Age 38(2):41. https://doi.org/10.1007/s11357-016-9885-2
Campbell AS, Ho CCG, Atık M, Allen M, Lincoln S, Malphrus K, Nguyen T, Oatman SR et al (2022) Clinical deep phenotyping of ABCA7 mutation carriers. Neurol Genet 8(2):e655. https://doi.org/10.1212/nxg.0000000000000655
Engelman CD, Koscik RL, Jonaitis EM, Okonkwo OC, Hermann BP, La Rue A, Sager MA (2013) Interaction between two cholesterol metabolism genes influences memory: findings from the Wisconsin Registry for Alzheimer's Prevention. J Alzheimer’s Dis 36(4):749–757. https://doi.org/10.3233/jad-130482
Chang YT, Hsu SW, Huang SH, Huang CW, Chang WN, Lien CY, Lee JJ, Lee CC et al (2019) ABCA7 polymorphisms correlate with memory impairment and default mode network in patients with APOEε4-associated Alzheimer’s disease. Alzheimer’s Res Therapy 11(1):103. https://doi.org/10.1186/s13195-019-0563-3
Sinha N, Berg CN, Shaw A, Gluck MA (2020) ABCA7 genotype moderates the effect of aerobic exercise intervention on generalization of prior learning in healthy older African Americans. J Alzheimer’s Dis 74(1):309–318. https://doi.org/10.3233/jad-190723
Berg CN, Sinha N, Gluck MA (2019) ABCA7 risk genotype diminishes the neuroprotective value of aerobic fitness in healthy older African Americans. Front Aging Neurosci 11:73. https://doi.org/10.3389/fnagi.2019.00073
Xi J, Ding D, Zhao Q, Liang X, Zheng L, Guo Q, Hong Z, Fu H et al (2020) Joint effect of ABCA7 rs4147929 and body mass index on progression from mild cognitive impairment to Alzheimer’s disease: the Shanghai Aging Study. Curr Alzheimer Res 17(2):185–195. https://doi.org/10.2174/1567205017666200317095608
Yu L, Ji H, Zhou M, Guo Y, Liu J, Lei D, Han C, Ma T (2022) ABCA7 rs3764650 polymorphism is associated with delayed neurocognitive recovery. Pharmacogenomics and Pers Med 15:301–309. https://doi.org/10.2147/pgpm.S352810
Pini L, Wennberg AM, Salvalaggio A, Vallesi A, Pievani M, Corbetta M (2021) Breakdown of specific functional brain networks in clinical variants of Alzheimer’s disease. Ageing Res Rev 72:101482. https://doi.org/10.1016/j.arr.2021.101482
Frisoni GB, Fox NC, Jack CR Jr, Scheltens P, Thompson PM (2010) The clinical use of structural MRI in Alzheimer disease. Nat Rev Neurol 6(2):67–77. https://doi.org/10.1038/nrneurol.2009.215
Ramirez LM, Goukasian N, Porat S, Hwang KS, Eastman JA, Hurtz S, Wang B, Vang N et al (2016) Common variants in ABCA7 and MS4A6A are associated with cortical and hippocampal atrophy. Neurobiol Aging 39:82–89. https://doi.org/10.1016/j.neurobiolaging.2015.10.037
Wachinger C, Nho K, Saykin AJ, Reuter M, Rieckmann A (2018) A longitudinal imaging genetics study of neuroanatomical asymmetry in Alzheimer’s disease. Biol Psychiatry 84(7):522–530. https://doi.org/10.1016/j.biopsych.2018.04.017
Shulman JM, Chen K, Keenan BT, Chibnik LB, Fleisher A, Thiyyagura P, Roontiva A, McCabe C et al (2013) Genetic susceptibility for Alzheimer disease neuritic plaque pathology. JAMA Neurol 70(9):1150–1157. https://doi.org/10.1001/jamaneurol.2013.2815
Apostolova LG, Risacher SL, Duran T, Stage EC, Goukasian N, West JD, Do TM, Grotts J et al (2018) Associations of the top 20 Alzheimer disease risk variants with brain amyloidosis. JAMA Neurol 75(3):328–341. https://doi.org/10.1001/jamaneurol.2017.4198
Ma FC, Zong Y, Wang HF, Li JQ, Cao XP, Tan L (2018) ABCA7 genotype altered Aβ levels in cerebrospinal fluid in Alzheimer’s disease without dementia. Ann Transl Med 6(22):437. https://doi.org/10.21037/atm.2018.07.04
Dong L, Mao C, Liu C, Li J, Huang X, Wang J, Lei D, Chu S et al (2022) Association between common variants of APOE, ABCA7, A2M, BACE1, and cerebrospinal fluid biomarkers in Alzheimer’s disease: data from the PUMCH Dementia Cohort. J Alzheimer’s Dis 85(4):1511–1518. https://doi.org/10.3233/jad-215067
Lyssenko NN, Praticò D (2021) ABCA7 and the altered lipidostasis hypothesis of Alzheimer’s disease. Alzheimers Dement 17(2):164–174. https://doi.org/10.1002/alz.12220
Sinha N, Reagh ZM, Tustison NJ, Berg CN, Shaw A, Myers CE, Hill D, Yassa MA et al (2019) ABCA7 risk variant in healthy older African Americans is associated with a functionally isolated entorhinal cortex mediating deficient generalization of prior discrimination training. Hippocampus 29(6):527–538. https://doi.org/10.1002/hipo.23042
Zhang XY, Wang YF, Zheng LJ, Zhang H, Lin L, Lu GM, Zhang LJ (2020) Impacts of AD-related ABCA7 and CLU variants on default mode network connectivity in healthy middle-age adults. Front Mol Neurosci 13:145. https://doi.org/10.3389/fnmol.2020.00145
Kao YC, Ho PC, Tu YK, Jou IM, Tsai KJ (2020) Lipids and Alzheimer’s disease. Int J Mol Sci 21(4). https://doi.org/10.3390/ijms21041505
Di Paolo G, Kim TW (2011) Linking lipids to Alzheimer’s disease: cholesterol and beyond. Nat Rev Neurosci 12(5):284–296. https://doi.org/10.1038/nrn3012
Claes C, Danhash EP, Hasselmann J, Chadarevian JP, Shabestari SK, England WE, Lim TE, Hidalgo JLS et al (2021) Plaque-associated human microglia accumulate lipid droplets in a chimeric model of Alzheimer’s disease. Molecular neurodegeneration 16(1):50. https://doi.org/10.1186/s13024-021-00473-0
Mapstone M, Cheema AK, Fiandaca MS, Zhong X, Mhyre TR, MacArthur LH, Hall WJ, Fisher SG et al (2014) Plasma phospholipids identify antecedent memory impairment in older adults. Nat Med 20(4):415–418. https://doi.org/10.1038/nm.3466
Casanova R, Varma S, Simpson B, Kim M, An Y, Saldana S, Riveros C, Moscato P et al (2016) Blood metabolite markers of preclinical Alzheimer’s disease in two longitudinally followed cohorts of older individuals. Alzheimers Dement 12(7):815–822. https://doi.org/10.1016/j.jalz.2015.12.008
Abe-Dohmae S, Yokoyama S (2021) ABCA7 links sterol metabolism to the host defense system: molecular background for potential management measure of Alzheimer's disease. Gene 768:145316. https://doi.org/10.1016/j.gene.2020.145316
Sakae N, Liu CC, Shinohara M, Frisch-Daiello J, Ma L, Yamazaki Y, Tachibana M, Younkin L et al (2016) ABCA7 deficiency accelerates amyloid-β generation and Alzheimer’s Neuronal Pathology. J Neurosci 36(13):3848–3859. https://doi.org/10.1523/jneurosci.3757-15.2016
Aikawa T, Ren Y, Holm ML, Asmann YW, Alam A, Fitzgerald ML, Bu G, Kanekiyo T (2021) ABCA7 regulates brain fatty acid metabolism during LPS-induced acute inflammation. Front Neurosci 15:647974. https://doi.org/10.3389/fnins.2021.647974
Hayashi M, Abe-Dohmae S, Okazaki M, Ueda K, Yokoyama S (2005) Heterogeneity of high density lipoprotein generated by ABCA1 and ABCA7. J Lipid Res 46(8):1703–1711. https://doi.org/10.1194/jlr.M500092-JLR200
Tomioka M, Toda Y, Mañucat NB, Akatsu H, Fukumoto M, Kono N, Arai H, Kioka N (1862) Ueda K (2017) Lysophosphatidylcholine export by human ABCA7. Biochimica et biophysica acta Molecular and cell biology of lipids 7:658–665. https://doi.org/10.1016/j.bbalip.2017.03.012
Picataggi A, Rodrigues A, Cromley DA, Wang H, Wiener JP, Garliyev V, Billheimer JT, Grabiner BC et al (1867) Lyssenko NN (2022) Specificity of ABCA7-mediated cell lipid efflux. Biochimica et biophysica acta Molecular and cell biology of lipids 7:159157. https://doi.org/10.1016/j.bbalip.2022.159157
Heath L, Earls JC, Magis AT, Kornilov SA, Lovejoy JC, Funk CC, Rappaport N, Logsdon BA et al (2022) Manifestations of Alzheimer's disease genetic risk in the blood are evident in a multiomic analysis in healthy adults aged 18 to 90. Sci Rep 12(1):6117. https://doi.org/10.1038/s41598-022-09825-2
Liu Y, Thalamuthu A, Mather KA, Crawford J, Ulanova M, Wong MWK, Pickford R, Sachdev PS et al (2021) Plasma lipidome is dysregulated in Alzheimer's disease and is associated with disease risk genes. Transl Psychiatry 11(1):344. https://doi.org/10.1038/s41398-021-01362-2
Haass C, Kaether C, Thinakaran G, Sisodia S (2012) Trafficking and proteolytic processing of APP. Cold Spring Harb Perspect Med 2(5):a006270. https://doi.org/10.1101/cshperspect.a006270
Leyns CEG, Holtzman DM (2017) Glial contributions to neurodegeneration in tauopathies. Mol Neurodegener 12(1):50. https://doi.org/10.1186/s13024-017-0192-x
Haass C, Koo EH, Mellon A, Hung AY, Selkoe DJ (1992) Targeting of cell-surface beta-amyloid precursor protein to lysosomes: alternative processing into amyloid-bearing fragments. Nature 357(6378):500–503. https://doi.org/10.1038/357500a0
Kim WS, Li H, Ruberu K, Chan S, Elliott DA, Low JK, Cheng D, Karl T et al (2013) Deletion of Abca7 increases cerebral amyloid-β accumulation in the J20 mouse model of Alzheimer’s disease. J Neurosci 33(10):4387–4394. https://doi.org/10.1523/jneurosci.4165-12.2013
Chishti MA, Yang DS, Janus C, Phinney AL, Horne P, Pearson J, Strome R, Zuker N et al (2001) Early-onset amyloid deposition and cognitive deficits in transgenic mice expressing a double mutant form of amyloid precursor protein 695. J Biol Chem 276(24):21562–21570. https://doi.org/10.1074/jbc.M100710200
Barbero-Camps E, Fernández A, Martínez L, Fernández-Checa JC, Colell A (2013) APP/PS1 mice overexpressing SREBP-2 exhibit combined Aβ accumulation and tau pathology underlying Alzheimer’s disease. Hum Mol Genet 22(17):3460–3476. https://doi.org/10.1093/hmg/ddt201
O'Connor T, Sadleir KR, Maus E, Velliquette RA, Zhao J, Cole SL, Eimer WA, Hitt B et al (2008) Phosphorylation of the translation initiation factor eIF2alpha increases BACE1 levels and promotes amyloidogenesis. Neuron 60(6):988–1009. https://doi.org/10.1016/j.neuron.2008.10.047
LaFerla FM, Green KN, Oddo S (2007) Intracellular amyloid-beta in Alzheimer’s disease. Nat Rev Neurosci 8(7):499–509. https://doi.org/10.1038/nrn2168
Qian XH, Song XX, Liu XL, Chen SD, Tang HD (2021) Inflammatory pathways in Alzheimer's disease mediated by gut microbiota. Ageing Res Rev 68:101317. https://doi.org/10.1016/j.arr.2021.101317
Jehle AW, Gardai SJ, Li S, Linsel-Nitschke P, Morimoto K, Janssen WJ, Vandivier RW, Wang N et al (2006) ATP-binding cassette transporter A7 enhances phagocytosis of apoptotic cells and associated ERK signaling in macrophages. J Cell Biol 174(4):547–556. https://doi.org/10.1083/jcb.200601030
Nowyhed HN, Chandra S, Kiosses W, Marcovecchio P, Andary F, Zhao M, Fitzgerald ML, Kronenberg M et al (2017) ATP binding cassette transporter ABCA7 regulates NKT cell development and function by controlling CD1d expression and lipid raft content. Sci Rep 7:40273. https://doi.org/10.1038/srep40273
Sh Y, Liu B, Zhang J, Zhou Y, Hu Z, Zhang X (2021) Application of artificial intelligence modeling technology based on fluid biopsy to diagnose Alzheimer’s disease. Front Aging Neurosci 13:768229. https://doi.org/10.3389/fnagi.2021.768229
Munawara U, Catanzaro M, Xu W, Tan C, Hirokawa K, Bosco N, Dumoulin D, Khalil A et al (2021) Hyperactivation of monocytes and macrophages in MCI patients contributes to the progression of Alzheimer’s disease. Immun Ageing 18(1):29. https://doi.org/10.1186/s12979-021-00236-x
Aikawa T, Holm ML, Kanekiyo T (2018) ABCA7 and pathogenic pathways of Alzheimer’s disease. Brain Sci 8(2). https://doi.org/10.3390/brainsci8020027
Aikawa T, Ren Y, Yamazaki Y, Tachibana M, Johnson MR, Anderson CT, Martens YA, Holm ML et al (2019) ABCA7 haplodeficiency disturbs microglial immune responses in the mouse brain. Proc Natl Acad Sci U S A 116(47):23790–23796. https://doi.org/10.1073/pnas.1908529116
Gosselet F, Candela P, Sevin E, Berezowski V, Cecchelli R, Fenart L (2009) Transcriptional profiles of receptors and transporters involved in brain cholesterol homeostasis at the blood-brain barrier: use of an in vitro model. Brain Res 1249:34–42. https://doi.org/10.1016/j.brainres.2008.10.036
Lamartinière Y, Boucau MC, Dehouck L, Krohn M, Pahnke J, Candela P, Gosselet F, Fenart L (2018) ABCA7 downregulation modifies cellular cholesterol homeostasis and decreases amyloid-β peptide efflux in an in vitro model of the blood-brain barrier. J Alzheimer’s Dis 64(4):1195–1211. https://doi.org/10.3233/jad-170883
Acknowledgements
Not applicable.
Funding
The authors gratefully acknowledge the financial supports by National Natural Science Foundation of China [81971014] and Shanghai Municipal Commission of Health and Family Planning [20184Y0056].
Author information
Authors and Affiliations
Contributions
Tang HD and Liu XL designed the study and prepared the manuscript. Qian XH and Chen SY summarized the research progress. All of the authors discussed the results and approved the manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
All authors agreed with the submitted version of the manuscript.
Competing Interests
The author declares no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qian, Xh., Chen, Sy., Liu, Xl. et al. ABCA7-Associated Clinical Features and Molecular Mechanisms in Alzheimer’s Disease. Mol Neurobiol 60, 5548–5556 (2023). https://doi.org/10.1007/s12035-023-03414-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03414-8