Abstract
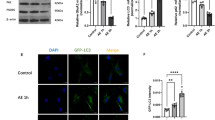
Memory loss, often known as amnesia, is common in the elderly population and refers to forgetting facts and experiences. It is associated with increased mitochondrial fragmentation, though the contribution of mitochondrial dynamics in amnesia is poorly understood. Therefore, the present study is aimed at elucidating the role of Mdivi-1 in mitochondrial dynamics, hippocampal plasticity, and memory during scopolamine (SC)-induced amnesia. The findings imply that Mdivi-1 significantly increased the expression of Arc and BDNF proteins in the hippocampus of SC-induced amnesic mice, validating improved recognition and spatial memory. Moreover, an improved mitochondrial ultrastructure was attributed to a decline in the percentage of fragmented and spherical-shaped mitochondria after Mdivi-1 treatment in SC-induced mice. The significant downregulation of p-Drp1 (S616) protein and upregulation of Mfn2, LC3BI, and LC3BII proteins in Mdivi-1-treated SC-induced mice indicated a decline in fragmented mitochondrial number and healthy mitochondrial dynamics. Mdivi-1 treatment alleviated ROS production and Caspase-3 activity and elevated mitochondrial membrane potential, Vdac1 expression, ATP production, and myelination, resulting in reduced neurodegeneration in SC mice. Furthermore, the decline of pro-apoptotic protein cytochrome-c and increase of anti-apoptotic proteins Procaspase-9 and Bcl-2 in Mdivi-1-treated SC-induced mice suggested improved neuronal health. Mdivi-1 also increased the dendritic arborization and spine density, which was further corroborated by increased expression of synaptophysin and PSD95. In conclusion, the current study suggests that Mdivi-1 treatment improves mitochondrial ultrastructure and function through the regulation of mitochondrial dynamics. These changes further improve neuronal cell density, myelination, dendritic arborization, and spine density, decrease neurodegeneration, and improve recognition and spatial memory.
Graphical Abstract
Schematic presentation depicts that Mdivi-1 rescues memory decline in scopolamine-induced amnesic male mice by ameliorating mitochondrial dynamics and hippocampal plasticity









Similar content being viewed by others
Data Availability
The data that support the findings of this study are available on the request from the corresponding author.
Abbreviations
- AD:
-
Alzheimer’s disease
- ANOVA:
-
Analysis of variance
- ATP:
-
Adenosine triphosphate
- cDNA:
-
Complementary DNA
- CNS:
-
Central nervous system
- Cyt-c:
-
Cytochrome-c
- Drp1:
-
Dynamin-related protein 1
- EDTA:
-
Ethylenediaminetetraacetic acid
- Fis1:
-
Mitochondrial fission 1 protein
- H2O2 :
-
Hydrogen peroxide
- HRP:
-
Horseradish peroxidase
- LTP:
-
Long-term potentiation
- M:
-
Mitochondria
- Mdivi-1:
-
Mitochondrial division inhibitor-1
- Mfn:
-
Mitofusin
- MMP:
-
Mitochondrial membrane potential
- NOR:
-
Novel object recognition
- PBS:
-
Phosphate-buffered saline
- PCR:
-
Polymerase chain reaction
- PD:
-
Parkinson’s disease
- PMSF:
-
Phenylmethanesulfonyl fluoride
- RDV:
-
Relative density value
- ROS:
-
Reactive oxygen species
- SC:
-
Scopolamine
- SEM:
-
Standard error of the mean
- SOD:
-
Superoxide dismutase
- TEM:
-
Transmission electron microscope
- Tris:
-
2-Amino-2-(hydroxymethyl) propane-1,3,-diol
- qRT-PCR:
-
Quantitative real-time PCR
References
Mattson MP, Magnus T (2006) Ageing and neuronal vulnerability. Nat Rev Neurosci 7:278–294. https://doi.org/10.1038/nrn1886
Wang W, Zhao F, Ma X, Perry G, Zhu X (2020) Mitochondria dysfunction in the pathogenesis of Alzheimer’s disease: recent advances. Mol Neurodegener 15:01–22. https://doi.org/10.1186/s13024-020-00376-6
Li S, Xiong GJ, Huang N, Sheng ZH (2020) The cross-talk of energy sensing and mitochondrial anchoring sustains synaptic efficacy by maintaining presynaptic metabolism. Nat Metab 2:1077–1095. https://doi.org/10.1038/s42255-020-00289-0
Li Z, Okamoto KI, Hayashi Y, Sheng M (2004) The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell 119:873–887. https://doi.org/10.1016/j.cell.2004.11.003
Stowers RS, Megeath LJ, Górska-Andrzejak J, Meinertzhagen IA, Schwarz TL (2002) Axonal transport of mitochondria to synapses depends on milton, a novel drosophila protein. Neuron 36:1063–1077. https://doi.org/10.1016/s0896-6273(02)01094-2
Messina F, Cecconi F, Rodolfo C (2020) Do you remember mitochondria? Front Physiol 11:271. https://doi.org/10.3389/fphys.2020.00271
Picard M, McEwen BS (2014) Mitochondria impact brain function and cognition. Proc Natl Acad Sci 111:7–8. https://doi.org/10.1073/pnas.1321881111
Olesen MA, Torres AK, Jara C, Murphy MP, Tapia Rojas C (2020) Premature synaptic mitochondrial dysfunction in the hippocampus during aging contributes to memory loss. Redox Biol 34:101558. https://doi.org/10.1016/j.redox.2020.101558
Martins IV, Rivers Auty J, Allan SM, Lawrence CB (2017) Mitochondrial abnormalities and synaptic loss underlie memory deficits seen in mouse models of obesity and Alzheimer’s disease. J Alzheimers Dis 55:915–932. https://doi.org/10.3233/JAD-160640
Giacomello M, Pyakurel A, Glytsou C, Scorrano L (2020) The cell biology of mitochondrial membrane dynamics. Nat Rev Mol Cell Biol 21:204–224. https://doi.org/10.1038/s41580-020-0210-7
Baloyannis SJ (2006) Mitochondrial alterations in Alzheimer’s disease. J Alzheimers Dis 9:119–126. https://doi.org/10.3233/jad-2006-9204
Qi X, Qvit N, Su YC, Mochly Rosen D (2013) A novel drp1 inhibitor diminishes aberrant mitochondrial fission and neurotoxicity. J Cell Sci 126:789–802. https://doi.org/10.1242/jcs.114439
Mishra E, Thakur MK (2022) Alterations in hippocampal mitochondrial dynamics are associated with neurodegeneration and recognition memory decline in old male mice. Biogerontology 23:251–271. https://doi.org/10.1007/s10522-022-09960-3
Wang W, Yin J, Ma X, Zhao F, Siedlak SL, Wang Z, Torres S, Fujioka H et al (2017) Inhibition of mitochondrial fragmentation protects against Alzheimer’s disease in rodent model. Hum Mol Genet 26:4118–4131. https://doi.org/10.1093/hmg/ddx299
Wang X, Su BO, Lee HG, Li X, Perry G, Smith MA, Zhu X (2009) Impaired balance of mitochondrial fission and fusion in Alzheimer’s disease. J Neurosci 29:9090–9103. https://doi.org/10.1523/jneurosci.1357-09.2009
Kiryu-Seo S, Tamada H, Kato Y, Yasuda K, Ishihara N, Nomura M, Mihara K, Kiyama H (2016) Mitochondrial fission is an acute and adaptive response in injured motor neurons. Sci Rep 6:28331. https://doi.org/10.1038/srep28331
Sheng Y, Yang G, Casey K, Curry S, Oliver M, Han SM, Leeuwenburgh C, Xiao R (2021) A novel role of the mitochondrial iron-sulfur cluster assembly protein ISCU-1/ISCU in longevity and stress response. Geroscience 43:691–707. https://doi.org/10.1007/s11357-021-00327-z
Kim H, Lee JY, Park KJ, Kim WH, Roh GS (2016) A mitochondrial division inhibitor, mdivi-1, inhibits mitochondrial fragmentation and attenuates kainic acid-induced hippocampal cell death. BMC Neurosci 17:1–10. https://doi.org/10.1186/s12868-016-0270-y
Yoboue ED, Valente EM (2020) Pink1 and parkin: the odd couple. Neurosci Res 159:25–33. https://doi.org/10.1016/j.neures.2020.04.007
Neuspiel M, Zunino R, Gangaraju S, Rippstein P, McBride H (2005) Activated mitofusin 2 signals mitochondrial fusion, interferes with Bax activation, and reduces susceptibiliradical-inducednduced depolarization. J Biol Chem 280:25060–25070. https://doi.org/10.1074/jbc.M501599200
Olichon A, Baricault L, Gas N, Guillou E, Valette A, Belenguer P, Lenaers G (2003) Loss of opa1 perturbates the mitochondrial inner membrane structure and integrity, leading to cytochrome c release and apoptosis. J Biol Chem 278:7743–7746. https://doi.org/10.1074/jbc.C200677200
Sugioka R, Shimizu S, Tsujimoto Y (2004) Fzo1, a protein involved in mitochondrial fusion, inhibits apoptosis. J Biol Chem 279:52726–52734. https://doi.org/10.1074/jbc.M408910200
Frank S, Gaume B, Bergmann-Leitner ES, Leitner WW, Robert EG, Catez F, Smith CL, Youle RJ (2001) The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell 1:515–525. https://doi.org/10.1016/S1534-5807(01)00055-7
Jagasia R, Grote P, Westermann B, Conradt B (2005) Drp-1-mediated mitochondrial fragmentation during egl-1-induced cell death in C. elegans. Nature 433:754–760. https://doi.org/10.1038/nature03316
Gan X, Huang S, Wu L, Wang Y, Hu G, Li G, Zhang H, Yu H et al (2014) Inhibition of ERK-DLP1 signaling and mitochondrial division alleviates mitochondrial dysfunction in Alzheimer’s disease cybrid cell. Biochim Biophys Acta Mol Basis Dis 1842:220–231. https://doi.org/10.1016/j.bbadis.2013.11.009
Blokland A (2005) Scopolamine-induced deficits in cognitive performance: a review of animal studies. Scopol Rev 1:01–76
Gautam A, Wadhwa R, Thakur MK (2013) Involvement of hippocampal arc in amnesia and its recovery by alcoholic extract of ashwagandha leaves. Neurobiol Learn Mem 106:177–184. https://doi.org/10.1016/j.nlm.2013.08.009
Baghel MS, Thakur MK (2017) Differential proteome profiling in the hippocampus of amnesic mice. Hippocampus 27:845–859. https://doi.org/10.1002/hipo.22735
Zhang G, Meng L, Wang Z, Peng Q, Chen G, Xiong J, Zhang Z (2022) Islet amyloid polypeptide cross-seeds tau and drives the neurofibrillary pathology in Alzheimer’s disease. Mol Neurodegener 17:01–18. https://doi.org/10.1186/s13024-022-00518-y
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Baghel MS, Thakur MK (2019) Vdac1 downregulation causes mitochondrial disintegration leading to hippocampal neurodegeneration in scopolamine-induced amnesic mice. Mol Neurobiol 56:1707–1718. https://doi.org/10.1007/s12035-018-1164-z
Prajapati SK, Garabadu D, Krishnamurthy S (2017) Coenzyme-Q10 prevents mitochondrial dysfunction and facilitates pharmacological activity of atorvastatin in 6-OHDA-induced dopaminergic toxicity in rats. Neurotox Res 31:478–492. https://doi.org/10.1007/s12640-016-9693-6
Mishra A, Krishnamurthy S (2019) Rebamipide mitigates impairments in mitochondrial function and bioenergetics with α-synuclein pathology in 6- OHDA-induced hemiparkinson’s model in rats. Neurotox Res 35:542–562. https://doi.org/10.1007/s12640-018-9983-2
Griffiths DE, Houghton RL (1974) Studies on energy-linked reactions: modified mitochondrial ATPase of oligomycin-resistant mutants of Saccharomyces cerevisiae. Eur J Biochem 46:157–167. https://doi.org/10.1111/j.1432-1033.1974.tb03608.x
Tiwari SK, Agarwal S, Chauhan LKS, Mishra VN, Chaturvedi RK (2015) Bisphenol-a impairs myelination potential during development in the hippocampus of the rat brain. Mol Neurobiol 51:1395–1416. https://doi.org/10.1007/s12035-014-8817-3
Zaqout S, Kaindl AM (2016) Golgi-cox staining step by step. Front Neuroanat 10:38. https://doi.org/10.3389/fnana.2016.00038
Titus ADJ, Shankaranarayana Rao BS, Harsha HN, Ramkumar K, Srikumar BN, Singh SB, Chattarji S, Raju TR (2007) Hypobaric hypoxia-induced dendritic atrophy of hippocampal neurons is associated with cognitive impairment in adult rats. Neuroscience 145:265–278. https://doi.org/10.1016/j.neuroscience.2006.11.037
Gensel JC, Schonberg DL, Alexander JK, McTigue DM, Popovich PG (2010) Semi-automated sholl analysis for quantifying changes in growth and differentiation of neurons and glia. J Neurosci Methods 190:71–79. https://doi.org/10.1016/j.jneumeth.2010.04.026
Xu B, He Y, Liu L, Ye G, Chen L, Wang Q, Chen M, Chen Y et al (2021) The effects of physical running on dendritic spines and amyloid-beta pathology in 3xTg-AD male mice. Aging Dis 13:1293–1310. https://doi.org/10.14336/AD.2022.0110
Wu M, Zhang M, Yin X, Chen K, Hu Z, Zhou Q, Cao X, Chen Z et al (2021) The role of pathological tau in synaptic dysfunction in Alzheimer’s diseases. Transl Neurodegener 10:01–11. https://doi.org/10.1186/s40035-021-00270-1
Müller WE, Eckert A, Kurz C, Eckert GP, Leuner K (2010) Mitochondrial dysfunction: common final pathway in brain aging and Alzheimer’s disease—therapeutic aspects. Mol Neurobiol 41:159–171. https://doi.org/10.1007/s12035-010-8141-5
Haam J, Yakel JL (2017) Cholinergic modulation of the hippocampal region and memory function. J Neurochem 142:111–121. https://doi.org/10.1111/jnc.14052
Nickel M, Gu C (2018) Regulation of central nervous system myelination in higher brain functions. Neural plast 2018:6436453. https://doi.org/10.1155/2018/6436453
Luo F, Herrup K, Qi X, Yang Y (2017) Inhibition of Drp1 hyper-activation is protective in animal models of experimental multiple sclerosis. Exp Neurol 292:21–34. https://doi.org/10.1016/j.expneurol.2017.02.015
Liu X, Song L, Yu J, Huang F, Li Y, Ma C (2022) Mdivi-1: a promising drug and its underlying mechanisms in the treatment of neurodegenerative diseases. Histol Histopathol 37:505–512. https://doi.org/10.14670/HH-18-443
Kim M-S, Lee DY, Lee J, Kim HW, Sung SH, Han J-S, Jeon WK (2018) Terminalia chebula extract prevents scopolamine-induced amnesia via cholinergic modulation and anti-oxidative effects in mice. BMC Complement Altern Med 18(1):1–11. https://doi.org/10.1186/s12906-018-2212-y
Newman L, Gold P (2016) Attenuation in rats of impairments of memory by scopolamine, a muscarinic receptor antagonist, by mecamylamine, a nicotinic receptor antagonist. Psychopharmacology 233:925–932. https://doi.org/10.1007/s00213-015-4174-9
Falsafi SK, Deli A, Höger H, Pollak A, Lubec G (2012) Scopolamine administration modulates muscarinic, nicotinic and NMDA receptor systems. Plos One 7:e32082. https://doi.org/10.1371/journal.pone.0032082
Konar A, Shah N, Singh R, Saxena N, Kaul SC, Wadhwa R, Thakur MK (2011) Protective role of ashwagandha leaf extract and its component Withanone on scopolamine-induced changes in the brain and brain-derived cells. Plos One 6:e27265. https://doi.org/10.1371/journal.pone.0027265
Muhammad T, Ali T, Ikram M, Khan A, Alam SI, Kim MO (2019) Melatonin rescue oxidative stress-mediated neuroinflammation/neurodegeneration and memory impairment in scopolamine-induced amnesia mice model. J Neuroimmune Pharmacol 14:278–294. https://doi.org/10.1007/s11481-018-9824-3
Dong Y, Digman MA, Brewer GJ (2019) Age-and AD-related redox state of NADH in subcellular compartments by fluorescence lifetime imaging microscopy. Geroscience 41:51–67. https://doi.org/10.1007/s11357-019-00052-8
Memudu AE, Adewumi AE (2021) Alpha lipoic acid ameliorates scopolamine-induced memory deficit and neurodegeneration in the cerebello-hippocampal cortex. Metab Brain Dis 36:1729–1745. https://doi.org/10.1007/s11011-021-00720-9
Cho B, Choi SY, Cho HM, Kim HJ, Sun W (2013) Physiological and pathological significance of dynamin-related protein 1 (Drp1)-dependent mitochondrial fission in the nervous system. Exp Neurobiol 22:149–157. https://doi.org/10.5607/en.2013.22.3.149
Ayanga BA, Badal SS, Wang Y, Galvan DL, Chang BH, Schumacker PT, Danesh FR (2016) Dynamin–related protein 1 deficiency improves mitochondrial fitness and protects against progression of diabetic nephropathy. J Am Soc Nephrol 27:2733–2747. https://doi.org/10.1681/ASN.2015101096
Filichia E, Hoffer B, Qi X, Luo Y (2016) Inhibition of drp1 mitochondrial translocation provides neural protection in dopaminergic system in a Parkinson’s disease model induced by MPTP. Sci Rep 6:1–13. https://doi.org/10.1038/srep32656
Ishihara N, Nomura M, Jofuku A, Kato H, Suzuki SO, Masuda K, Otera H, Nakanishi Y et al (2009) Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nat Cell Biol 11:958–966. https://doi.org/10.1038/ncb1907
Aishwarya R, Alam S, Abdullah CS, Morshed M, Nitu SS, Panchatcharam M, Miriyala S, Kevil CG et al (2020) Pleiotropic effects of Mdivi-1 in altering mitochondrial dynamics, respiration, and autophagy in cardiomyocytes. Redox Biol 36:101660. https://doi.org/10.1016/j.redox.2020.101660
Penner M, Roth T, Chawla M, Hoang L, Roth E, Lubin F, Sweatt J, Worley P et al (2011) Age-related changes in Arc transcription and DNA methylation within the hippocampus. Neurobiol Aging 32:2198–2210. https://doi.org/10.1016/j.neurobiolaging.2010.01.009
Srivas S, Thakur MK (2017) Epigenetic regulation of neuronal immediate early genes is associated with decline in their expression and memory consolidation in scopolamine-induced amnesic mice. Mol Neurobiol 54:5107–5119. https://doi.org/10.1007/s12035-016-0047-4
Srivas S, Thakur MK (2018) Transcriptional co-repressor sin 3a silencing rescues decline in memory consolidation during scopolamine-induced amnesia. J Neurochem 145:204–216. https://doi.org/10.1111/jnc.14320
Nikolaienko O, Patil S, Eriksen MS, Bramham CR (2018) Arc protein: a flexible hub for synaptic plasticity and cognition. In Seminars in cell & developmental biology. Semin Cell Dev Biol 77:33–42. https://doi.org/10.1016/j.semcdb.2017.09.006
Lee J-S, Kim H-G, Lee H-W, Han J-M, Lee S-K, Kim D-W, Saravanakumar A, Son C-G (2015) Hippocampal memory enhancing activity of pine needle extract against scopolamine-induced amnesia in a mouse model. Sci Rep 5:1–10. https://doi.org/10.1038/srep09651
Ruiz A, Alberdi E, Matute C (2018) Mitochondrial division inhibitor 1 (Mdivi-1) protects neurons against excitotoxicity through the modulation of mitochondrial function and intracellular ca2+ signaling. Front Mol Neurosci 11:3. https://doi.org/10.3389/fnmol.2018.00003
Taguchi N, Ishihara N, Jofuku A, Oka T, Mihara K (2007) Mitotic phosphorylation of dynamin-related GTPase drp1 participates in mitochondrial fission. J Biol Chem 282:11521–11529. https://doi.org/10.1074/jbc.M607279200
Zhao F, Wang W, Wang C, Siedlak SL, Fujioka H, Tang B, Zhu X (2017) Mfn2 protects dopaminergic neurons exposed to paraquat both in vitro and in vivo: implications for idiopathic Parkinson’s disease. Biochim Biophys Acta Mol Basis Dis 1863:1359–1370. https://doi.org/10.1016/j.bbadis.2017.02.016
Alaimo A, Gorojod RM, Beauquis J, Munoz MJ, Saravia F, Kotler ML (2014) Deregulation of mitochondria-shaping proteins Opa-1 and Drp-1 in manganese-induced apoptosis. Plos One 9:e91848. https://doi.org/10.1371/journal.pone.0091848
Ko AR, Hyun HW, Min SJ, Kim JE (2016) The differential drp1 phosphorylation and mitochondrial dynamics in the regional-specific astroglial death induced by status epilepticus. Front Cell Neurosci 10:124. https://doi.org/10.3389/fncel.2016.00124
Grohm J, Kim S, Mamrak U, Tobaben S, Cassidy-Stone A, Nunnari J (2012) Inhibition of drp1 provides neuroprotection in vitro and in vivo. Cell Death Differ 19:1446–1458. https://doi.org/10.1038/cdd.2012.18
Cribbs JT, Strack S (2007) Reversible phosphorylation of drp1 by cyclic amp-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO Rep 8:939–944. https://doi.org/10.1038/sj.embor.7401062
Martorell-Riera A, Segarra-Mondejar M, Muñoz JP, Ginet V, Olloquequi J, Pérez-Clausell J, Palacín M, Reina M et al (2014) Mfn2 downregulation in excitotoxicity causes mitochondrial dysfunction and delayed neuronal death. EMBO J 33:2388–2407. https://doi.org/10.15252/embj.201488327
Jiang S, Nandy P, Wang W, Ma X, Hsia J, Wang C, Wang Z, Niu M et al (2018) Mfn2 ablation causes an oxidative stress response and eventual neuronal death in the hippocampus and cortex. Mol Neurodegener 13:1–15. https://doi.org/10.1186/s13024-018-0238-8
Rappold PM, Cui M, Grima JC, Fan RZ, de Mesy-Bentley KL, Chen L, Zhuang X, Bowers WJ et al (2014) Drp1 inhibition attenuates neurotoxicity and dopamine release deficits in vivo. Nat Commun 5:1–13. https://doi.org/10.1038/ncomms6244
Han S, Zhang M, Jeong YY, Margolis DJ, Cai Q (2021) The role of mitophagy in the regulation of mitochondrial energetic status in neurons. Autophagy 17:4182–4201. https://doi.org/10.1080/15548627.2021.1907167
Mishra E, Thakur MK (2023) Mitophagy: a promising therapeutic target for neuroprotection during ageing and age-related diseases. Br J Pharmacol 180:1542–1561. https://doi.org/10.1111/bph.16062
Fang EF, Hou Y, Palikaras K, Adriaanse BA, Kerr JS, Yang B, Lautrup S, Hasan-Olive MM et al (2019) Mitophagy inhibits amyloid-β and tau pathology and reverses cognitive deficits in models of Alzheimer’s disease. Nat Neurosci 22:401–412. https://doi.org/10.1038/s41593-018-0332-9
Bido S, Soria FN, Fan RZ, Bezard E, Tieu K (2017) Mitochondrial division inhibitor-1 is neuroprotective in the a53t-α-synuclein rat model of Parkinson’s disease. Sci Rep 7:1–13. https://doi.org/10.1038/s41598-017-07181-0
Nandi A, Yan LJ, Jana CK, Das N (2019) Role of catalase in oxidative stress-and age-associated degenerative diseases. Oxid Med Cell Longev 2019:9613090. https://doi.org/10.1155/2019/9613090
Attaluri S, Arora M, Madhu LN, Kodali M, Shuai B, Melissari L, Upadhya R, Rao X et al (2022) Oral nano-curcumin in a model of chronic gulf war illness alleviates brain dysfunction with modulation of oxidative stress, mitochondrial function, neuroinflammation, neurogenesis, and gene expression. Aging Dis 13:583. https://doi.org/10.14336/AD.2021.0829
Bordt EA, Zhang N, Waddell J, Polster BM (2022) The non-specific drp1 inhibitor mdivi-1 has modest biochemical antioxidant activity. Antioxidants 11:450. https://doi.org/10.3390/antiox11030450
Wong KY, Roy J, Fung ML, Heng BC, Zhang C, Lim LW (2020) Relationships between mitochondrial dysfunction and neurotransmission failure in Alzheimer’s disease. Aging Dis 11:1291. https://doi.org/10.14336/AD.2019.1125
Rhein V, Song X, Wiesner A, Ittner LM, Baysang G, Meier F, Ozmen L, Bluethmann H et al (2009) Amyloid-β and tau synergistically impair the oxidative phosphorylation system in triple transgenic Alzheimer’s disease mice. Proc Natl Acad Sci 106:20057–20062. https://doi.org/10.1073/pnas.0905529106
David DC, Hauptmann S, Scherping I, Schuessel K, Keil U, Rizzu P, Ravid R, Dröse S et al (2005) Proteomic and functional analyses reveal a mitochondrial dysfunction in p301l tau transgenic mice. J Biol Chem 280:23802–23814. https://doi.org/10.1074/jbc.M500356200
Wu Q, Gao C, Wang H, Zhang X, Li Q, Gu Z, Shi X, Cui Y et al (2018) Mdivi-1 alleviates blood-brain barrier disruption and cell death in experimental traumatic brain injury by mitigating autophagy dysfunction and mitophagy activation. Int J Biochem Cell Biol 94:44–55. https://doi.org/10.1016/j.biocel.2017.11.007
Bordt EA, Clerc P, Roelofs BA, Saladino AJ, Tretter L, Adam-Vizi V, Cherok E, Khalil A et al (2017) The putative drp1 inhibitor mdivi-1 is a reversible mitochondrial complex I inhibitor that modulates reactive oxygen species. Dev Cell 40:583 594-e586. https://doi.org/10.1016/j.devcel.2017.02.020
Liu J, Zhao Y, Yang J, Zhang X, Zhang W, Wang P (2017) Neonatal repeated exposure to isoflurane not sevoflurane in mice reversibly impaired spatial cognition at juvenile-age. Neurochem Res 42:595–605. https://doi.org/10.1007/s11064-016-2114-7
Nickel M, Gu C (2018) Regulation of central nervous system myelination in higher brain functions. Neural Plast 2018:6436453. https://doi.org/10.1155/2018/6436453
Fields RD, Bukalo O (2020) Myelin makes memories. Nat Neurosci 23:469–470. https://doi.org/10.1038/s41593-020-0606-x
Papuć E, Rejdak K (2020) The role of myelin damage in Alzheimer’s disease pathology. Arch Med Sci 16:345. https://doi.org/10.5114/aoms.2018.76863
Lockrow JP, Fortress AM, Granholm ACE (2012) Age-related neurodegeneration and memory loss in down syndrome. Curr Gerontol Geriatr Res 2012:463909. https://doi.org/10.1155/2012/463909
Stadelmann C, Deckwerth TL, Srinivasan A, Bancher C, Brück W, Jellinger K, Lassmann H (1999) Activation of caspase-3 in single neurons and autophagic granules of granulovacuolar degeneration in Alzheimer’s disease: evidence for apoptotic cell death. Am J Pathol 155:1459–1466. https://doi.org/10.1016/S0002-9440(10)65460-0
D’Amelio M, Sheng M, Cecconi F (2012) Caspase-3 in the central nervous system: beyond apoptosis. Trends Neurosci 35:700–709. https://doi.org/10.1016/j.tins.2012.06.004
Kudryashova I, Onufriev M, Kudryashov I, Gulyaeva N (2009) Caspase-3 activity in hippocampal slices reflects changes in synaptic plasticity. Neurosci Behav Physiol 39:13–20. https://doi.org/10.1007/s11055-008-9089-z
Maletic-Savatic M, Malinow R, Svoboda K (1999) Rapid dendritic morphogenesis in ca1 hippocampal dendrites induced by synaptic activity. Science 283:1923–1927. https://doi.org/10.1126/science.283.5409.1923
Zhang Y, Rui T, Luo C, Li Q (2021) Mdivi-1 alleviates brain damage and synaptic dysfunction after intracerebral hemorrhage in mice. Exp Brain Res 239:1581–1593. https://doi.org/10.1007/s00221-021-06089-6
Ortiz-Sanz C, Gaminde-Blasco A, Valero J, Bakota L, Brandt R, Zugaza JL, Matute C, Alberdi E (2020) Early effects of aβ oligomers on dendritic spine dynamics and arborization in hippocampal neurons. Front Synaptic Neurosci 12:01–11. https://doi.org/10.3389/fnsyn.2020.00002
Bouton ME (2004) Context and behavioral processes in extinction. Learn Mem 11:485–494. http://www.learnmem.org/cgi/doi/10.1101/lm.78804
Acknowledgements
We are thankful to the Interdisciplinary School of Life Sciences (ISLS), BHU, for the Vibratome, Cryostat, qRT-PCR, and fluorescence microscopy facilities. We are also thankful to the TEM facility of Dr. Harisingh Gour Central University, Sagar, MP, India.
Funding
We acknowledge the Council of Scientific & Industrial Research (CSIR) for the Junior and Senior Research Fellowship and Indian Council of Medical Research (ICMR), New Delhi, for Senior Research Fellowship to EM, research grants from ICMR (5/4–5/153/Neuro/2015-NCD-I), Department of Science & Technology (EMR/2015/002178), Government of India, and National Academy of Sciences, India-Senior Scientist fellowship (NASI-303/12/2021) to MKT.
Author information
Authors and Affiliations
Contributions
EM: Conceptualization, Data curation, Formal Analysis, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing; MKT: Conceptualization, Funding acquisition, Investigation, Project administration, Resources, Supervision, Validation, Writing – final review & editing.
Corresponding author
Ethics declarations
Ethical Approval
The current study was approved by the Institutional Animal Ethical Committee (IAEC Approval Reference No. BHU/DoZ/IAEC/2018–19/043), and experimentation was carried out by following CPCSEA guidelines.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Message
• Mdivi-1 enhances recognition and spatial memory of amnesic mice.
• Mdivi-1 alters mitochondrial dynamics-related proteins and promotes mitochondrial health.
• Mdivi-1 improves mitochondrial function by altering SOD, Catalase, ROS, and ATP.
• Mdivi-1 enhances myelination and attenuates neurodegeneration in amnesic mice.
• Mdivi-1 augments the dendritic arborization, spine density, and hippocampal plasticity.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mishra, E., Thakur, M.K. Mdivi-1 Rescues Memory Decline in Scopolamine-Induced Amnesic Male Mice by Ameliorating Mitochondrial Dynamics and Hippocampal Plasticity. Mol Neurobiol 60, 5426–5449 (2023). https://doi.org/10.1007/s12035-023-03397-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03397-6