Abstract
Various neurotrophins (NTs), including nerve growth factor, brain-derived neurotrophic factor, neurotrophin-3, and neurotrophin-4, promote cellular differentiation, survival, and maintenance, as well as synaptic plasticity, in the peripheral and central nervous system. The function of microRNAs (miRNAs) and other small non-coding RNAs, as regulators of gene expression, is pivotal for the appropriate control of cell growth and differentiation. There are positive and negative loops between NTs and miRNAs, which exert modulatory effects on different signaling pathways. The interplay between NTs and miRNAs plays a crucial role in the regulation of several physiological and pathological brain procedures. Emerging evidence suggests the diagnostic and therapeutic roles of the interactions between NTs and miRNAs in several neuropsychological disorders, including epilepsy, multiple sclerosis, Alzheimer’s disease, Huntington’s disease, amyotrophic lateral sclerosis, schizophrenia, anxiety disorders, depression, post-traumatic stress disorder, bipolar disorder, and drug abuse. Here, we review current data regarding the regulatory interactions between NTs and miRNAs in neuropsychological disorders, for which novel diagnostic and/or therapeutic strategies are emerging. Targeting NTs-miRNAs interactions for diagnostic or therapeutic approaches needs to be validated by future clinical studies.
Similar content being viewed by others
Introduction
The mammalian neurotrophins (NTs), a family of structurally-related proteins, regulate neurite outgrowth and modulate neuronal differentiation and survival [1]. The NT family consists of four proteins: brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), neurotrophin-3 (NT3), and neurotrophin-4 (NT4), which are important in the regulation of various physiological and pathological conditions [2]. There are two different classes of receptors that are activated by NTs, (i) the p75 neurotrophin receptors (p75NTR), which are a low-affinity NTs receptors, and (ii) the tropomyosin-related kinase receptors (Trk), which are high-affinity NTs receptors and consist of TrkA, TrkB, and TrkC [3, 4]. NGF has the highest affinity to TrkA, BDNF and NT4 stimulate preferentially TrkB, and NT3 acts mainly through TrkC. Several diverse intracellular signaling pathways may be affected by the activation of Trk, including the phosphatidylinositol 3-kinase-protein kinase B (PI3K-Akt) signaling pathway, extracellular-signal-regulated kinase 1/2 (ERK1/2), PI3K, phospholipase C, and mitogen-activated protein kinase (Ras/Raf/MAPK) pathway [5,6,7,8]. The p75NTR is a multifunctional transmembrane protein, which can unselectively bind to all mature NTs [9]. The p75NTR couple to different intracellular binding proteins, activate signaling adaptors, and modulate the Trk signaling pathway. The p75NTR contributes to the neurogenesis of adult neural progenitors via NGF activation [10, 11]. Several signaling elements of the Trk pathways mediate NT functions, particularly gene transcription regulation [3]. Moreover, NTs are implicated in the regulation of several transcription factors (TFs), like cAMP response element-binding protein B (CREB) and nuclear factor kappa B (NF-κB) [12]. There are functional connections between NTs, TFs, and their transcriptional targets [13]. Enhancement of NT expression in the brain could protect neuronal tissues against a variety of pathological insults, such as ischemic and traumatic events as well as neurodegenerative processes [14, 15]. NTs and their receptors are also involved in neuropsychiatric disorders [16].
The bidirectional interactions between NTs and brain-specific microRNAs (miRNAs) regulate the expression of numerous protein-encoding genes [17]. miRNAs, a class of tiny non-coding RNAs with a length of approximately 22 nucleotides, are the major post-transcriptional regulators of gene expression [18]. miRNA precursors are located within both intragenic and intergenic regions of DNA. miRNAs function involves a multi-step process, including transcription and processing of primary miRNAs, precursor-miRNAs hairpin formation, and export of mature miRNAs from the cytoplasm to the nucleus [19, 20]. Multiple factors play a regulatory role in these processes, including RNA polymerase II, rosha, Exportin 5, Dicer, and Argonaute [21]. Mature miRNA can be sorted and loaded into the Argonaute proteins to form an RNA-induced silencing complex [22].
Approximately 30–60% of all mammalian proteins can be targeted by miRNAs, which are implicated in various cellular and developmental processes. miRNAs regulate cell proliferation, differentiation, regeneration, and cell death [23, 24]. miRNAs and their abundant targets also play a pivotal role in neural lineage and subtype determination as well as neural stem cell development in both physiological and pathological states [25]. Circulating miRNAs are released into the extracellular fluids, such as blood, urine, and cerebrospinal fluid (CSF) [26]. Growing evidence suggests that brain-specific miRNAs play an important role in the regulation of neuronal activity [27]. Furthermore, miRNAs mediate neuronal communication via regulating the protein synthesis that is implicated in synaptic transmission [28]. miRNA dysregulation in the nervous system could affect a wide range of biological functions, such as neurogenesis, myelination, and dendritic outgrowth [29, 30]. Dysregulation in the miRNA signaling disrupts the functions of neurons [31], astrocytes [32], microglia [33], oligodendrocytes [30], and ependymal cells [34]. miRNAs are implicated as biologically crucial mediators in the pathogenesis of various neuropsychological diseases, such as Alzheimer’s disease (AD), Huntington’s disease (HD), Parkinson’s disease (PD), epilepsy, anxiety, depression, schizophrenia, post-traumatic stress disorder (PTSD), bipolar disorder, and substance abuse [31, 35,36,37]. miRNAs could serve as a potential biomarker for the early detection of various neurodegenerative disorders [26].
Both NTs and brain-specific miRNAs play a potential role in diagnostic and therapeutic approaches for central nervous system (CNS) disorders. Indeed, a strong relationship between the regulation of NTs-miRNAs and the pathophysiology of various brain disorders has been determined [38,39,40]. Here, we summarize the current understanding of the regulatory mechanisms of NTs and miRNAs interactions. Furthermore, we provide a comprehensive review of the current knowledge regarding the potential diagnostic, predictive, prognostic, and/or therapeutic roles of the NTs and miRNAs interactions in neuropsychological disorders.
Molecular Interactions between NTs and miRNA Signaling
miRNAs are considered one of the important regulators in eukaryotic transcription [41]. Various brain-specific miRNAs are known to play a critical role in NT expression and function [31]. NTs and miRNAs mutually regulate each other. NT expression is not only regulated by miRNAs, but it, in turn, modulates miRNA expression [42, 43]. NTs are involved in a wide range of gene expressions mostly at the level of transcription and translation [44]. NTs modulate MAPK/ERK pathways and control miRNA levels. The modulation of MAPK/ERK could alter miRNA values through (i) phosphorylation of TAR RNA binding protein and Dicer, (ii) regulation of CREB and NF-κB, and (iii) alteration of Lin-28 homolog A [17]. A given miRNA may directly regulate multiple mRNAs, each of them has different binding sites to promote the binding performance [45].
Effect of miRNAs on NT Expression
Brain-enriched miRNAs have been described to play a critical role in NT expression. NTs regulate neuronal and synaptic functions during development and adulthood, and miRNAs modulate NTs [46,47,48]. miRNAs target different mRNAs through the interaction with the 3′ untranslated regions (3′ UTR); however, binding to other regulatory regions of mRNA can also occur [43, 49]. The direct or indirect interactions between miRNAs and their target genes can be influenced by multiple factors [50]. Some miRNAs mediate NTs expression through post-transcriptionally regulating TFs expression. CREB, as a transcription factor (TF), stimulates transcription in association with CREB-binding protein and its homolog p300. CREB could also bind to various BDNF promoter elements and enhance the NT activities [17, 41, 51]. For instance, miR-134 directly targets CREB mRNA and inhibits its translation. Inhibition of CREB signaling could abolish the BDNF expression [17].
miRNAs modulate NTs in an isoform-specific manner. An in vitro study on the human neuroblastoma cell line SH-SY5Y has shown that an isoform of the neurotrophic receptor tyrosine kinase 3 (NTR3) is specifically regulated by different sets of miRNAs. Overexpression of miR-128 regulates the truncated isoform of NTR3 (t-NTR3) and miR-151-3p regulates the full-length isoform of NTR3 at the mRNA level [46]. In vitro analysis of TrkC expression in retinoic acid (RA)-treated SK-N-BE cells indicated that t-NTR3 mRNA can be targeted by miR-9, miR-125a, and miR-125b. A regulatory circuitry involving these miRNAs and TrkC has been identified to play a key role in controlling cell proliferation [52]. miRNAs can also regulate NTs receptors under certain conditions. Upregulation of P75NTR is implicated in the pathogenesis of brain injury and apoptosis. miR-592 could regulate p75NTR at the mRNA level, and an inverse relationship is defined between miR-592 and p75NTR [17, 53]. We have identified various miRNAs that can directly target NTs in humans and mice using the miRTarBase database (Table 1).
miRNAs Interactions with NGF, BDNF, and NT3
miRNAs can modulate the expression of NGF, BDNF, and NT3. Multiple studies have revealed the role of miRNA downstream on NGF to regulate cell proliferation and/or apoptosis and consequently modulate neuronal differentiation [54]. miR-200 inhibits cell proliferation and promotes cell differentiation and neurite formation via targeting TF SRY-box transcription factor 2 and kruppel-like factor 4 [55]. Studies on developing rat brains as well as pheochromocytoma cell lines (PC12) provide supporting evidence that miR-29a and miR-29c increase neurite outgrowth through direct inhibition of tumor suppressor gene phosphatase and tensin homolog expression [56]. In PC12 cells, miR-200 targets some TFs and induces a neural marker, neurofilament light polypeptide [55, 57]. Previous studies have also demonstrated that miR-183 and/or miR-96 inhibit NGF-treated PC12 differentiation [58]. Furthermore, miR-221 plays a pivotal role in the NGF signaling, and overexpression of miR-221 can replace NGF in neural differentiation and survival [59]. miR-21 maintains the NGF effect on neuronal survival [60].
miR-155 upregulation enhances NGF expression at the protein level and its downregulation inhibits cytokine signaling 1 expression and NF-κB activation (Table 2) [61]. Knockout of miR-204/211 increases NGF expression at the mRNA level and activates the Akt signaling pathway (Table 2) [62]. miR-455-3p can also directly target NGF mRNA [63]. Another study suggests that lethal (Let)-7-5p is an upstream regulator of NGF [64]. In vivo studies elucidate that let-7 and miR-675 can directly target P53 and NGF mRNA [65,66,67]. Transfection of human dorsal root ganglia cell culture with miR-455-3p significantly reduced NGF expression at the mRNA level, which was reversible after the application of a miR-455-3p inhibitor (Table 2) [68]. Moreover, downregulation of miR-125b reversely increases NGF expression at the mRNA and protein levels (Table 2) [69]. Chronic inflammatory pain leads to the upregulated expression of miR-29b, which promotes the demethylation at the promoter region of the NGF gene, resulting in the upregulation of NGF gene expression (Table 2) [70]. Analysis of CSF of patients infected with acute viral encephalitis has shown overexpression of miR-150-5p that negatively correlated with transforming growth factor-β, NGF, axon guidance, and MAPK [71]. An experimental study on an acute cerebral ischemia model indicates that upregulation of miR-381 inhibits leucine-rich repeat containing-4 via the stromal cell-derived factor-1/C-X-C chemokine receptor type 4 signaling, enhances NGF protein expression, prevents neuronal apoptosis (Table 2) [72].
Both miRNAs and BDNF play a role in the regulation of brain synaptic plasticity. Deletion of various brain-specific miRNAs, such as miR-124, miR-132, miR-137, miR-138, miR29a, and miR29c, increased hippocampal synaptic transmission as well as the expression of BDNF protein. Different miRNAs, such as miR-15a, miR-206, and miR-210, directly regulate BDNF protein expression and activity [12, 73]. Moreover, several investigations revealed that BDNF mRNA is the direct target of miR-10b, miR-19, miR-22, miR-26a-1, miR-26a-2, miR-26b, miR-195, and miR-30a-5p [74,75,76]. miR-15a has been suggested to inhibit the proliferation of neuronal cells and promote cell apoptosis by targeting BDNF mRNA and protein through downregulation of the PI3K/AKT pathway [77]. The high expression of miR-153 by targeting leptin receptors significantly suppresses the Janus kinase/signal transducers and activators of the transcription signaling pathway and thereby enhances BDNF expression at the mRNA and protein levels and neuronal proliferation (Table 2) [78]. Overexpression of miR-10a by targeting the BDNF signaling pathway could inhibit cell proliferation and induce neuronal apoptosis in the hippocampus [79]. Upregulation of miR-211 significantly inhibits BDNF mRNA and protein expression and suppresses the viability and proliferation of normal human astrocytes via the activation of lipopolysaccharides and the PI3K/Akt pathway (Table 2) [80]. Methyl CpG binding protein 2 (MeCP2) is one of the important genes in the maturation of new neurons and its dysregulation plays a role in the pathophysiology of Rett syndrome [81].
MeCP2 is a transcriptional regulator of BDNF [82]. The BDNF protein level was reduced in the MeCP2 mutant mice and an increase in BDNF levels improved motor skills in both mutant mice and children with Rett syndrome [83]. It was demonstrated that deletion of BDNF in MeCP2 mutations caused an earlier onset of overt symptoms in patients with Rett syndrome [84]. Several investigations provide evidence regarding the functional interaction between MeCP2 and BDNF. The modulation of BDNF pathways has been suggested as a potential strategy for treating children with Rett syndrome [85]. MeCP2 also regulates miR-15a and its reduction leads to abnormality in dendrite morphology during neurogenesis. On the other hand, miR-15a regulates BDNF expression at the mRNA and protein levels and exogenous BDNF can partially compensate for miR-15a deficiency during neuron maturation [86].
BDNF and miR-124 play a critical role in the pathogenesis of acute ischemic stroke. Contrary to other investigations, a negative correlation has been observed between serum BDNF and miR-124 values in patients with ischemic stroke [87]. Several miRNAs might act as diagnostic, prognostic, and/or therapeutic biomarkers for human gliomas [88]. A negative regulatory correlation between miR-103 and the BDNF mRNA and protein expression levels has been reported in gliomas. Overexpression of miR-103 inhibits the proliferation and invasion of cancer cells in patients with gliomas through downregulation of BDNF (Table 2) [89]. The oxygen–glucose deprivation/reoxygenation enhances miR-1 expression, which directly suppresses the expression of BDNF mRNA and protein, and subsequently affects cell survival and apoptosis [90]. Downregulation of miR-134 reduces ischemic injury through upregulation of CREB and downstream genes, including BDNF and Bcl-2, in ischemic hippocampal neurons (Table 2) [91].
Experimental evidence indicates that alterations of miRNAs contribute to neuropathic pain [92]. Using in vitro model of chronic constriction injury, it has been shown that decreased miR-30a-3p contributes to neuropathic pain. This study suggested that miR-30a-3p may inhibit BDNF activation via targeting the acetylated histone H3 and H4 on its promoter (Table 2) [93]. Investigations on animal and human embryonic stem cell (hESC)-derived neurons have revealed the association between anesthesia-induced neural injury and increasing hsa-miR-375 and miR-170 levels. These studies indicated that the BDNF gene is directly and reversely regulated by hsa-miR-375 and miR-170 and its upregulation protects neurons from anesthesia-induced neuronal cell damage and neural toxicity [94, 95]. The reduction of miR-21 level in a mice model of the spinal cord injury led to the upregulation of BDNF gene expression (Table 2) [96].
Resveratrol, a potent silent information regulator 1 (Sirt1), downregulates miR-134 and consequently causes an increase in CREB/BDNF expression levels in the hippocampus and improves hippocampal-dependent learning and memory [97]. In chronic alcohol-treated animals, downregulation of miR-183c in association with overexpression of BDNF mRNA exhibits a neuroprotective effect (Table 2) [98]. Overexpression of miR-124 in Schwann cells significantly enhances the BDNF and NT3 mRNA expression, which might involve in neuron development processes (Table 2) [99]. NT3 and BDNF are predictive targets for miR-182 upregulation that might negatively control NT3 and BDNF expression in ancestral stress-induced behaviors [100]. miR-30c transfection can also improve neuronal injury and increase NT3 and BDNF expression in the rat hippocampus (Table 2) [101]. miR-200c and miR-429 also directly target NT3 mRNA [102, 103].
miRNA Interactions with TrkA, TrkB, TrkC, and P75NTR
Several miRNAs regulate the TrkA, TrkB, TrkC, and P75NTR signaling pathways [104]. Alterations in the NGF/TrkA signaling pathway are important in neuroblastoma cell differentiation and regression. On the other hand, alteration of miR-92a expression levels is related to the biological behavior of neuroblastoma cells. Higher miR-92a expression values increase the proliferation and migration of human neuroblastoma cells via downregulation of TrkA [105]. The impact of miRNAs on TrkB was evaluated using SHSY5Y cells. miR-216b regulates TrkB-Shc through binding to 3’ UTR [104]. A study on early brain injury after subarachnoid hemorrhage indicated that the administration of human umbilical mesenchymal stem cells-derived miR-206-knockdown exosomes impedes brain injury via the modulation of the BDNF/TrkB/CREB signaling pathway [106].
The results of the human genetic analysis revealed that the expression of miR-185 may impact neurodevelopment through the regulation of the NTR3 gene [47, 107]. Furthermore, CNS damage can target p75NTR and lead to neuronal apoptosis and cell death. Reduction in miR-592, a key regulator of p75NTR, modulates neuronal injury and reduces cell apoptosis after ischemic insults [108]. Furthermore, miR-18a downregulates TrkA and p75NTR mRNA levels in neuroblastoma cell culture [109]. Experimental studies have shown that miR-141 binds to NGF receptor-associated protein 1 mRNA and suppresses the NGF/p75NTR signaling (Table 2) [110].
Effect of NTs on miRNA Expression
NTs regulate the expression of miRNAs through the activation of various specific TFs, such as NF-kB and CREB. Activation of Trk receptors leads to upregulation of the ERK/CREB signaling pathway, which is involved in the regulation of primiR-212/132 transcription [12]. Besides, the activation of p75NTR can lead to the activation of the NF-kB pathway [111]. The activation of the ERK1/2 and CREB signaling pathways implicated in the NGF-induced expression of miRNAs can promote NGF-related cell survival. For instance, NGF induces miR-221/222 expression through the activation of the ERK1/2 pathway and results in a reduction of pro-apoptosis protein and cell survival (Tables 3 and 4) [112].
In PC12 cells, sustained mitogen-activated protein kinase/ERK activity and activation of transcription factor activator protein 1 in response to NGF, positively regulate miR-21, which plays an important role in brain development. miR-21 also contributes to the activation of the NGF signaling [113]. Treatment of PC12 cells with NGF modulates the expression of miR-29c, miR-93, miR-212/132, miR-103, miR-30c, miR-691, miR-207, and miR-709 [60]. NGF causes an increase of synapsin I expression via downregulation of miR-541, which plays a crucial role in neurite outgrowth and particularly localizes in axon membrane (Tables 3 and 4) [114]. The proliferation and terminal differentiation of neuronal cells are regulated by the NGF receptor, TrkA, as well as by downstream signaling cascades, including Ras–MAPK, PI3K–Akt pathways, and inositol triphosphate-mediated calcium release [115]. An in vivo study on hypersensitive bladder demonstrates that increased NGF expression is associated with upregulation and downregulation of miR-132 and miR-221, respectively (Table 3) [116]. BDNF is the main regulator of neuron survival with an inhibitory effect on neuronal apoptosis through the activation of the PI3K/Akt pathway. It has been suggested that dysregulation of BDNF-miRNA interaction could result in apoptosis [90].
NT Receptor Signaling Interaction with miRNAs
NGF is one of the key modulators of miRNA [59, 60]. The PC12 cell line has been used as a model for the study of the interaction between NGF and miRNAs expressions. Treatment of PC12 cells with NGF upregulates the expressions of miR-34, miR-181a, miR-200, and miR-326 and downregulates the expressions of miR-106b, miR-126, miR-139-3p, miR-143, miR-210, and miR-532-3p (Table 3, 4) [59]. An in vitro study on the role of NGF in the proliferation of human corneal cells revealed that NGF downregulates miR-494 and thereby restores its direct target, Cyclin D, a protein required for the progression of the G1 phase of the cell cycle (Tables 3 and 4) [117]. NGF also downregulates miR-23b by a TF named c-Myc [118]. In primary sensory neurons, NGF inhibits miR-181d-mediated suppression of microtubule-associated protein 1B and calmodulin and consequently leads to axonal elongation (Table 3 and 4) [119]. Differentiation of NGF-treated Muller cells toward neurons is associated with the inhibition of miR-98 as well as Let-7b, Let-7d, and Let-7i (Tables 3 and 4) [120]. Moreover, NGF induces miR-34a expression via the inhibition of tumor suppressor P53 and maintains mature neural cells in the G1 phase (Table 3, 4) [54]. NGF deprivation suppresses miR-21 levels which consequently leads to the elevation of cell division cycle 25 homolog A, caspase activation, and neural death (Tables 3 and 4) [121].
miRNAs can be also regulated by BDNF. It has been reported that miR-1 is dysregulated after BDNF gene deletion in neurons of the dorsal root ganglion [122]. Furthermore, BDNF increases the ratio of miR-212/132 in cortical neuron culture. These miRNAs are regulated by the ERK pathway and particularly downstream effectors mitogen and stress-activated kinase1 and CREB (Tables 3 and 5) [12]. Several studies have found that increased expression of miR-29 controls the upregulation of miR-145 following BDNF-induced SH-SY5Y cell differentiation [123]. miR-134 inhibits LIM kinase-1 (LIMK1) which is crucial for the size of a dendritic spine. BDNF prevents the inhibitory effect of miR-134 on LIMK1 expression and maintains synaptic plasticity [124]. miR-125b is involved in neuroblastoma cell differentiation. RA and BDNF promote miR-125b expression and increase neurite outgrowth (Tables 3 and 5) [125]. In cortical neuron cell culture, BDNF upregulates miR-132 expression via the MAPK/ERK1/2 pathway and leads to increased neurite growth. Interestingly, downregulation of the MAPK/ERK pathway inhibits the BDNF-dependent increase of miR-132. The increase of miR-132 expression through MAPK/ERK1/2 is essential for BDNF-dependent overexpression of postsynaptic proteins, particularly N-methyl-D-aspartate 2A and glutamate receptor 1 (Tables 3 and 5) [12, 126].
The interaction between BDNF and miR-140 plays a key role in astrocyte proliferation following an injury to the spinal cord [127]. Furthermore, regulatory interaction between BDNF and miRNAs could modulate the proliferation of cancer cells. After treatment with cisplatin, a higher miR-16 expression associated with greater BDNF levels significantly reduces cancer cell differentiation and growth [128]. Radiotherapy on abdomen malignancies by affecting gut flora results in cognitive impairments. miR-34a-5p upregulation in the small intestine and peripheral blood leads to BDNF reduction in the hippocampus and subsequently cognitive dysfunction. Intravenous injection of miR-34a-5p antagomir can prevent gut flora changes and cognitive abnormalities (Table 2) [129]. Moreover, BDNF improves cell survival and inhibits apoptosis in hypoxic-hypoglycemic hippocampal neurons through the activation of TrkB and the inhibition of miR-134 expression. This BDNF effect could be mediated through the modulation of the TrkB/miR-134 pathway (Tables 3 and 5) [130].
The Role of NTs-miRNA Interaction in Neuropsychological Disorders
NTs-miRNAs interplay plays a key role in the modulation of neural regeneration as well as in cognitive functions [131, 132]. Various studies have suggested that circulating miRNAs can serve as early diagnosis and prognostic biomarkers in neurodegenerative and neuropsychiatric diseases [45, 133]. The mRNA levels of BDNF, NT4, and specific miRNA in peripheral blood mononuclear cells (PBMC) could potentially serve as biomarkers of CNS inflammation and neurodegenerative processes [134]. In the following, we will discuss the role of NTs-miRNA interactions in different neuropsychiatric disorders.
Role of NTs-miRNA Interaction in Various Neurological Diseases
Epilepsy
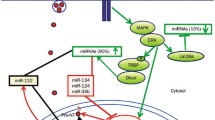
Dysregulation of NTs-miRNAs interaction is involved in the pathogenesis of epilepsy [135]. Targeting NTs-miRNAs interaction affects several biological processes and could be a strategy for efficient intervention following a potential epileptogenic insult [135, 136]. The BDNF/TrkB signaling plays a modulatory role in the brain’s dynamic state leading to greater excitability of mesial temporal lobe epilepsy (MTLE) [137, 138]. An enhancement of the BDNF/TrkB signaling, mediated via BDNF overexpression in the hippocampus, contributes to epileptogenesis in MTLE [139]. Moreover, overexpression of miR-155 significantly suppressed the BDNF and TrkB protein expression and exhibited a neuroprotective effect on epilepsy-induced neuronal damage via the PI3K/Akt/mammalian target of rapamycin (mTOR) signaling pathway in patients with MTLE (Fig. 1) [140]. Application of miR-155 antagonist significantly enhanced the expression of BDNF both at mRNA and protein levels in an animal MTLE model and resulted in the reduction of epileptiform burst discharges and seizure-like behaviors (Table 2, Fig. 1) [141]. Furthermore, upregulation of miR-132 promotes epileptogenesis via the BDNF-TrkB signaling in the primary cultures of hippocampal neurons (Fig. 1) [142]. Inhibition of miR-103a suppresses astrocyte activation in the hippocampus and improves neuronal injury by downregulation of the BDNF gene (Table 2, Fig. 1) [143]. miR-21 expression in the hippocampus is significantly increased after seizures. Enhancement of miR-21 is associated with a decrease in the inhibitory effect of NT3 and an increase in neuronal apoptosis. A significant enhancement of the expression of passenger strand miR-21 compared to mature miR-21 has been observed in the rat hippocampus after pilocarpine-induced status epilepticus. An inverse relationship has been observed between miR-21 and NT3 mRNA levels in hippocampal neurons after status epileptic. Targeting of NT3 by mature miR-21 could potentially result in a greater transforming growth factor-beta receptor expression and contributes to epileptogenesis [144,145,146]. A higher expression of miR-21 could downregulate the expression of NT3 (Table 2, Fig. 1) [143, 147]. Therefore, miR-21 has been suggested as an ideal target for the modulation of the NT3 signaling in the hippocampus following status epilepticus [146].
Multiple Sclerosis
The dysregulation of NTs-miRNAs interaction may influence the inflammatory process in multiple sclerosis (MS) [148]. However, limited investigations were designed to investigate the interaction between NTs and miRNAs in MS [149]. It has been indicated that different NTs, such as BDNF, might produce within active MS lesions [150]. BDNF receptors have been found in reactive astrocytes and neurons in active MS plaques [151]. NGF can improve axon regeneration, synaptogenesis, cell survival, oligodendrocyte differentiation, and oligodendrocyte precursor proliferation in the sclerosis plaques. NGF also promotes the production of BDNF and regulates key proteins essential for myelination [152]. In the experimental autoimmune encephalomyelitis model, it has been shown that BDNF mRNA correlated negatively with pro-inflammatory miR-155-5p expression levels (Fig. 1) [153]. Studies conducted on PBMCs obtained from patients with MS have revealed a decrease in BDNF mRNA expression and simultaneous increases in miR-132 and miR-182-5p values (Fig. 1) [134]. Moreover, the expression of miR-125a, miR-146b, and miR-200c significantly increased, while the expression of miR-328, miR-199a, and miR-152 markedly decreased in peripheral blood of patients with MS [135, 151]. BDNF mRNA was identified as a target of miR-99b and miR-125a. Interestingly, an inverse association was reported between miR-125a and BDNF in an experimental MS model (Fig. 1) [153]. In MS lesions, autoimmune and mesenchymal stem cells protect specific cell populations and suppress the formation of new lesions through the release of NGF and GDNF at the lesion sites [154]. Various types of microglia play complex roles in neuroinflammation and regeneration processes in MS [155]. miR-142-3p is one of the highly upregulated miRNAs in microglia in response to various pathological insults, such as the inflammatory process in MS [156]. miR-142-3p modulates BDNF expression via its target calcium/calmodulin-dependent kinase 2a and modulates the expression of proinflammatory mediators (Fig. 1) [157].
HD
The underlying molecular mechanisms of HD, such as alterations in synaptic plasticity, gene expression, neurotransmitter signaling, NTs, and miRNAs, can be considered potential therapeutic targets [131]. The abnormal expression of global miRNAs or specific miRNAs has been determined in different regions of the brains of HD-affected subjects [131, 158]. The striatum has been identified as a primary site of degeneration in HD. It has been hypothesized that altered BDNF delivery from the neocortex to the striatum plays a role in the pathophysiology of HD [159]. The decreased BDNF release and transport in neocortical neurons lead to insufficient trophic support of the striatum and enhance the vulnerability of striatal neurons and synapses in a knock-in mouse model of HD [160, 161].
A limited number of studies have investigated the effect of NTs-miRNA interaction in HD [131]. The modulatory interplay between miR-132, BDNF, and MeCP2 plays a key role in the pathophysiology of HD. miR-124 is positively regulated by BDNF in HD (Table 5) [162]. Furthermore, there is regulatory feedback between miR-132 and MeCP2, as well as with its downstream target BDNF in HD. Upregulation of miR-132 leads to the suppression of MeCP2 and BDNF transcript levels and consequently striatal cell death (Fig. 1) [17]. Furthermore, miRNA-124 may contribute to neurogenesis by regulating NTs in HD. miR-124 increases the value of BDNF, promotes neurogenesis, and improves neuronal survival in the striatum in an animal model of HD (Table 2, Fig. 1) [163]. Studies on the miRNA profile of patients with HD revealed upregulation of miR-10b-5p and miR-30a-5p, which leads to downregulation of BDNF and neuronal death. CREB1 is the predicted target gene of these two miRNAs in HD. miR-10b-5p has a neuroprotective effect in response to the mutation in HD (Fig. 1) [164]. Since HD is an inherited neurodegenerative condition through different mechanisms, such as abnormality and misfolding of proteins, mitochondrial dysfunctions, and degradation of misfolded protein, targeting NTs-miRNA interaction may provide a potential treatment option for HD [165, 166]. Moreover, it has been shown that the p65 subunit of NF-κB regulates miR-146a in an HD experimental model [167].
AD
miRNAs play an important regulatory role in different neurodegenerative diseases [36]. BDNF is crucial to the maintenance of neocortical network activities and its dysfunction contributes to memory impairment in AD [168]. NGF also plays a key role in the maintenance of neural structural integrity and function and enhances cell survival and regeneration in subjects with age-related diseases, such as AD [169]. The epigenetic mechanisms, like DNA methylation and miRNA alterations, can regulate the expression of NTs in patients with AD [131]. An elevated value of different miRNAs in human prefrontal neocortical tissue was associated with a reduced value of BDNF [170].
miRNAs are gene modulatory molecules with neuroprotective roles in the development of AD. The levels of different miRNAs are associated with the expression of various AD-related proteins [171]. Analysis of CSF and serum of patients with mild cognitive impairment and dementia of AD type, as well as the hippocampus of an AD mice model, indicated that miR-613 downregulates the expression of BDNF through directly targeting 3′ UTR (Table 2, 6, Fig. 1) [172]. Lower values of BDNF have been identified in the neocortex and hippocampus in AD [173]. CSF analysis of patients with AD indicated that the expression of miR-29c was positively associated with the protein expression of BDNF; suggesting its effect on neuronal proliferation through the regulation of BDNF expression (Fig. 1) [174]. Neuropeptide Y, a potent orexigenic neuromodulator in the brain, increased BDNF mRNA and protein expression by inhibiting miR-30a-5p in an in vitro model of AD (Fig. 1) [173, 175]. Increased miR-206 brain level has been observed in the mouse model of AD, whereas its reduction promoted the BDNF levels and improved cognitive functions (Fig. 1) [176]. miR-322 produces tau phosphorylation by negatively regulating BDNF-TrkB signal activation in AD (Fig. 1) [177]. Moreover, intraventricular application of amyloid-β1-42 (Aβ1-42) reduced the miR-107 level in mice. However, the administration of miR-107 mimic prevented the impairments of spatial memory and synaptic plasticity as well as the cell loss caused by Aβ neurotoxicity through the inhibition of the BDNF-TrkB signaling pathway (Fig. 1) [178]. An enhancement of miR-455-3p expression has been reported in patients with AD, which was associated with Aβ pathologies and modulation of NGF (Fig. 1) [179]. Moreover, miR-10a overexpression inhibits hippocampal synapse remodeling and cell proliferation and promotes apoptosis in AD rats through the inhibition of the BDNF-TrkB signaling pathway (Fig. 1) [180].
PD
miRNAs have been reported to implicate in pathways related to the pathophysiology of PD [181]. Previous studies indicated that downregulation of Dicer expression in dopamine neurons may cause dysregulation of various miRNAs linked to PD-associated genes, such as miR-133b, and regulates the function of aged dopaminergic neurons [182]. Furthermore, NTs are involved in multiple signaling cascades that play roles in the progression of PD pathology [183]. The BDNF/TrkB signaling is essential for the survival and maturation of the nigrostriatal dopaminergic neurons. The BDNF inhibits neuronal apoptosis and promotes the maturation of functional dopaminergic neurons [131, 184]. Upregulation of miR-34a, miR-141, and miR-9 is associated with downregulation of Sirt1, B-cell lymphoma protein 2, and BDNF mRNA in an in vitro PD model (Fig. 1). Importantly, this study has shown that miR-34a could become the target of the alteration of human BDNF levels for the treatment of PD (Table 2) [185].
In the PD neuron models, upregulation of miR-210-3p reduces BDNF production and results in neuronal damage (Table 2, Fig. 1) [186]. Elevated miR-21 levels and reduced peroxisome proliferator-activated receptor alpha (PPARα) values have been observed in patients with PD. A combined application of an omega-3 fatty acid and aspirin effectively promoted the expression of PPARα protein as well as BDNF and GDNF protein via the inhibition of miR-21 in SH-Y5Y cells (Fig. 1) [187]. miR-30e has significantly downregulated in the substantia nigra in a mouse model of PD. Application of the miR-30e agomir restored the sustained decreased BDNF production in these mice, which was associated with improved motor behavioral function and neural network activity (Fig. 1) [188]. Furthermore, the link between BDNF and various miRNAs, such as miR-210-3p, miR-34a, miR-141, miR-9, miR-21, and miR-30, in PD pathology, has been suggested [189]. A potential role of dysregulation of hypothalamic BDNF and miR-30e via the modulation of the melanocortin-4 receptor in the pathophysiology of PD has been suggested [131]. Moreover, miR-30a-5p reduces BDNF values and exerts a neurotoxic role on dopaminergic neurons in PD (Fig. 1) [190]. miR-7 also regulates the expression of BDNF through an autoregulatory mechanism in the early stages of neuronal damage in the atrazine-induced rat model of PD (Fig. 1) [191]. Alterations of miR-134 and miR-141 modulate the expression of mesencephalic astrocyte-derived neurotrophic factor and cerebral dopamine neurotrophic factor that play a role in the pathophysiology of several neurological disorders, including PD [192].
Amyotrophic Lateral Sclerosis
Enhancement of BDNF levels has been considered one of the main strategies to stop or prolong the progression of amyotrophic lateral sclerosis (ALS). The modulation of the BDNF/TrkB pathway under certain conditions exerts neuroprotective effects on motor neurons against various pathological insults [193,194,195], probably via the inhibition of apoptosis and restoring the impaired calcium homeostasis [196]. Dysregulation of several miRNAs, such as miR-132, miR-125b, miR-34a, and miR-504, has been determined in patients with ALS [197,198,199]. Microglia are a possible source of dysregulated miRNAs in ALS [200]. The dysfunction of microglial downregulates BDNF/TrkB signaling in motor neurons of ALS mice [201]. The inhibition of miR-125b exerts a neuroprotective effect on motor neurons via both reduction of pro-inflammatory mediators and the stimulation of microglia activators, such as BDNF, in ALS (Fig. 1) [197]. Different miRNAs, such as miR-320a, miR-424-5p, and miR-503, modulate the differentiation of mesenchymal stromal cells induced to express high levels of neurotrophic factors and potentially could be used as a biomarker in ALS clinical trials [202].
Role of miRNAs and NTs in Psychological Disorders
Based on the function of NTs in neuronal development and synaptic plasticity, a growing body of relevant evidence suggests the implication of NTs in the pathophysiology of various psychological disorders [203]. Furthermore, several studies have suggested that alterations of miRNAs expression profiles could contribute to the pathophysiology of psychological disorders, such as schizophrenia, depression, anxiety, drug abuse, PTSD, and bipolar disorder [204,205,206]. Besides, some studies indicate the importance of the NTs-miRNAs interactions in the development and progression of several neuropsychiatric disorders [207].
Depression
Alterations in the expression of different NTs contribute to the pathophysiology of depression [208, 209]. It has been suggested that the enhancement of the NTs signaling has a strong potential for the treatment of depression and the molecules-derived NTs pathways might be considered a biomarker for depression [210, 211]. BDNF exerts region-dependent antidepressant effects. Shati/Nat8l, an N-acetyltransferase in the dorsal striatum, can regulate BDNF via epigenetic regulations. The targeting of the Shati/Nat8l-BDNF pathway could be a potential therapeutic target for the treatment of depression [208]. Furthermore, it has been found that the level of NGF mRNA in the brain is correlated with anxiety and depression symptoms [212]. Evidence from experimental investigations and postmortem studies suggests that alterations of miRNAs contribute to the pathology of depression. Both upregulation and downregulation of several miRNAs have been reported in patients with depression [213]. miR-221 involves in the development of depression. It targets the wingless-type MMTV integration site family member 2, which results in the decreased activity of the CREB/BDNF signaling pathway in the hippocampus (Table 2, Fig. 1) [214]. miR-124 is a type of miRNA abundantly expressed in the hippocampus and directly targets the glucocorticoid receptor expression in the human embryonic kidney-293 cells. Downregulation of miR-124 may provide a strategy for the treatment of depression by activating the BDNF-TrkB, ERK, and CREB signaling pathways in the hippocampus. Under long-time exposure to stressful conditions, glucocorticoid hormones may cause depression via the regulatory effect of miR-124 on BDNF (Table 2, Fig. 1) [215]. In vivo study of corticosterone-induced depressive-like mice indicated that upregulation of miR-124 is required for the inhibition of the CREB-TrkB signaling pathway in the hippocampus [216]. Changes in miR-124a might participate in the induction of depressive-like behavior through direct regulation of BDNF gene expression in stressed rats (Fig. 1) [217]. Moreover, alterations in miR-132 and miR-124 values in non-treated and citalopram-treated patients with depression have shown that enhancement of both miRNAs increases plasma BDNF values (Fig. 1) [207].
Ketamine, a potent anti-depressive substance, decreases miR-206 expression in the hippocampus and miR-206 upregulation significantly reduces the ketamine-dependent increase of BDNF (Fig. 1) [218]. In maternal deprivation-induced depressive-like behaviors, overexpression of miR-16 is accompanied by a significant decrease in BDNF (Fig. 1) [204]. Furthermore, a decrease in BDNF levels was associated with increased values of miR-132 and miR-182 in patients with depression; suggesting a potential role of serum BDNF and its related miRNAs as diagnostic biomarkers (Table 2, Fig. 1) [219]. Upregulation of miR-202-3p significantly increases depressive-like behaviors, decreases the expression of BDNF, and reduces hippocampal damage in rats (Table 2, Fig. 1) [220]. Moreover, interactions between miR-132 and MeCP2 modulate the hippocampal BDNF protein expression in a rat model of chronic stress-induced depression [221]. Furthermore, miR-26a-3p plays a key role in the hippocampal neuronal network alterations in a chronic unpredictable mild stress (CUMS)-induced rat model through its regulatory effects on BDNF and the phosphatase/tensin homolog/PI3K/Akt signaling pathway [222]. In vitro neuronal studies and in vivo models of CUMS revealed that miR-182 directly inhibits BDNF and leads to lower CREB levels and depression-like behaviors (Table 2, Fig. 1) [223].
Anxiety Disorders
Experimental and clinical studies indicate the involvement of BDNF in anxiety disorders. Different types of stressors lead to a reduction of BDNF expression values [224]. Furthermore, several studies have reported the association of NTR3 activation with the pathophysiology of anxiety disorders. Consequently, it has been demonstrated that miR-9 and miR-125 regulate the expression of the t-NTR3 isoform in anxiety-like behaviors [225]. It has been reported that the reduction of miR-124a expression in the hippocampal dentate gyrus leads to decreased anxiety-like behavior, which is inversely correlated with the expression of its target gene, BDNF (Fig. 1) [226]. In the chronic unpredictable stress-induced depression rat model, miR-10b downregulation and BDNF upregulation have been shown in the hippocampus (Fig. 1) [75].
Schizophrenia
Both experimental and clinical investigations suggest that alterations in NTs and miRNAs in certain brain regions are implicated in the pathophysiology of schizophrenia [227, 228]. miR-137 regulates the expression of schizophrenia-associated genes and contributes to the regulation of neuronal response by targeting the PI3K-Akt-mTOR branch of neuregulin-1/ErbB and BDNF signaling [229]. In the prefrontal cortex of patients with schizophrenia, downregulation of neuropeptide Y and somatostatin mRNA values are associated with increased miR-195 levels and decreased BDNF expression (Fig. 1) [230]. The correlation between miR-195 and BDNF changes may play a role in GABAergic neurotransmission abnormalities and influence cognitive impairments of patients with schizophrenia [230, 231]. Furthermore, the alterations of the miR-30a family in the prefrontal cortex of patients with schizophrenia are associated with changes in BDNF levels (Fig. 1) [170, 232, 233]. Early growth response 3 (EGR3) and some miRNAs play a modulatory role in the schizophrenia regulatory neuronal network [234]. EGR3, a downstream gene of different signaling pathways, is triggered by various NTs, such as NGF and BDNF [235].
Substance Use Disorders
Dysregulation of various miRNAs-NTs interactions is implicated in the pathophysiology of drug abuse. There are growing studies that explore the interplay between drug abuse and NTs biological action in various brain regions. The expression of striatal BDNF correlates with the expression of CREB, TrkB, and pri-miR-132 following amphetamine application in rats [236]. Heavy alcohol use causes upregulation of some miRNAs and consequently regulates BDNF values [237]. Adolescent intermittent ethanol exposure increased the miR-137 expression level in the amygdala. Application of miR-137 antagomir in the amygdala decreased BDNF levels and improved anxiety-like behavior following alcohol consumption in rats (Fig. 1) [238]. Ethanol exposure decreased BDNF and enhanced miR-206 expression in different mice brain structures, including the medial prefrontal cortex, central amygdala, and hippocampus (Fig. 1) [239]. Induction of miR-206 expression and its modulation of BDNF after prolonged brain exposure to ethanol could alter the synaptic plasticity implicated in the cognitive control of alcohol consumption and lead to alcohol dependence [240]. In an investigation of brain tissue of alcohol-dependent rats, a significant alteration of miR-101b and BDNF expression has been reported [241]. Overexpression of miR-30a-5p in association with reduced levels of BDNF in the medial prefrontal cortex can play an important role in the transition from moderate to excessive alcohol intake (Fig. 1) [242]. Furthermore, ketamine-induced neural death and toxicity are accompanied by miR-375 upregulation that directly downregulates BDNF expression in hESC (Fig. 1) [94]. miR-206 downregulates BDNF levels in both neuronal cell culture in vitro and the hippocampus in vivo (Fig. 1) [243]. The interaction between miR-206 and BDNF expression in the nucleus accumbens has been suggested to control the reconsolidation of cocaine-associated memory [244]. Overexpression of miR-495 and its direct effect on BDNF value in the nucleus accumbens has been observed after acute cocaine administration in mice (Fig. 1) [245]. Alterations of miR-212 may regulate cocaine intake through the modulation of striatal CREB and MeCP2 signaling; which consequently decreases BDNF protein levels and decrease the motivational effects of cocaine (Fig. 1) [246,247,248]. Moreover, changes in miR-124, miR-181a, and let-7d as well as BNDF values in the mesolimbic dopaminergic system are implicated in a complex feedback loop with cocaine-induced plasticity [249].
PTSD
In a mouse model of PTSD, a strong reduction of miR-15a-5p, miR-497a-5p, miR-511-5p, and let-7d-5p levels in the medial prefrontal cortex were correlated with two key PTSD-related genes, FKBP5 and BDNF (Fig. 1) [250]. In another study on a rat model, it was suggested that miR-132 is involved in PTSD and led to the reduction of BDNF expression through MeCP2. The application of its antagomir can improve anxiety behavior and upregulates MeCP2 and BDNF (Fig. 1) [251]. Furthermore, rats exposed to stress exhibited enhanced miR-142-5p values in the amygdala, which was accompanied by a reduction in levels of Npas4, an activity-regulated transcription factor. The inhibition of miR-142-5p in these rats reduced anxiety-like behaviors and enhanced Npas4 and BDNF expressions (Fig. 1) [252].
Bipolar Disorder
An investigation of 288 patients with bipolar disorder revealed that interaction between miR-206 and BDNF polymorphism increases the risk for bipolar disorder and treatment response to various drugs [253]. Furthermore, it has been shown that the expression of peripheral miR-7-5p, miR-221-5p, and miR-370-3p are correlated to BDNF levels in 98 patients with bipolar patients (Fig. 1) [254]. Lithium is the major medication for mood stabilizing in bipolar disorder, which exhibits neuroprotective effects. Experiments on lithium pretreated SH-SY5Y human neuroblastoma cells provide evidence that lithium significantly decreases the expression of miR-34a, which is correlated with BDNF and anti-apoptotic protein BCL2 levels (Fig. 1) [255].
Conclusion
A series of experimental and clinical studies yields promising results suggesting the pivotal roles of NTs and miRNAs interactions in the pathophysiology of various neuropsychological disorders. Understanding how NTs and miRNAs interactions modulate pathological processes in different brain disorders helps design novel diagnostic and therapeutic approaches. Modulation of several brain-specific miRNAs could alter the expression and function of different NTs and vice versa. Changes in NTs and miRNAs expression and function contribute to brain hyperexcitability in epileptic patients, neurodegenerative processes in patients with AD, PD, HD, or ALS, demyelination in patients with MS, and perturbation in neural circuits and neurotransmitters in patients with different psychological disorders. On the other hand, alterations in NTs and miRNAs interactions could regulate neuronal and synaptic hyperexcitability, exert neuroprotective effects, promote myelination, and improve cognitive and behavioral impairment. Future studies are required to discover the exact mechanism of interplay between NTs and miRNAs in physiological and pathological conditions.
Data Availability
Not applicable.
Abbreviations
- AD:
-
Alzheimer’s disease
- ALS:
-
Amyotrophic lateral sclerosis
- BDNF:
-
Brain-derived neurotrophic factor
- CREB:
-
CAMP response element-binding protein B
- CNS:
-
Central nervous system
- CSF:
-
Cerebrospinal fluid
- CUMS:
-
Chronic unpredictable mild stress
- EGR3:
-
Early growth response 3
- ERK1/2:
-
Extracellular-signal-regulated kinase 1/2
- hESC:
-
Human embryonic stem cell
- HD:
-
Huntington’s disease
- LIMK1:
-
LIM kinase-1
- mTOR:
-
Mammalian target of rapamycin
- MeCP2:
-
Methyl CpG binding protein 2
- miRNAs:
-
MicroRNAs
- Ras/Raf/MAPK:
-
Mitogen-activated protein kinase
- MS:
-
Multiple sclerosis
- NGF:
-
Nerve growth factor
- NTs:
-
Neurotrophins
- NT3:
-
Neurotrophin-3
- NT4:
-
Neurotrophin-4
- NTR3:
-
Neurotrophic receptor tyrosine kinase 3
- NF-κB:
-
Nuclear factor kappa B
- PD:
-
Parkinson’s disease
- PC12:
-
Pheochromocytoma cell line
- PI3K-Akt:
-
Phosphatidylinositol 3-kinase-protein kinase B
- PBMCs :
-
Peripheral blood mononuclear cells
- PPARα:
-
Peroxisome proliferator-activated receptor alpha
- PTSD:
-
Post-traumatic stress disorder
- p75NTR:
-
P75 neurotrophin receptor
- RA:
-
Retinoic acid
- Sirt1:
-
Silent information regulator 1
- TF:
-
Transcription factor
- TFs:
-
Transcription factors
- Trk:
-
Tropomyosin-related kinase
- t-NTR3:
-
Truncated isoform of NTR3
- 3′ UTR:
-
3′ Untranslated region
References
Thomaz A, Jaeger M, Brunetto AL, Brunetto AT, Gregianin L, de Farias CB, Ramaswamy V, Nör C, Taylor MD et al (2020) Neurotrophin Signal Medulloblastoma Cancers 12:2542
László A, Lénárt L, Illésy L, Fekete A, Nemcsik J (2019) The role of neurotrophins in psychopathology and cardiovascular diseases: psychosomatic connections. J Neural Transm 126:265–278
Reichardt LF (2006) Neurotrophin-regulated signalling pathways. Philos. Trans. R. Soc. Lond., B. Biol Sci 361:1545–1564
Bothwell M (2016) Recent advances in understanding neurotrophin signaling. F1000Res 5:F1000 Faculty Rev-1885
Leopold AV, Chernov KG, Shemetov AA, Verkhusha VV (2019) Neurotrophin receptor tyrosine kinases regulated with near-infrared light. Nat Commun 10:1–3
Zanin JP, Montroull LE, Volosin M, Friedman WJ (2019) The p75 neurotrophin receptor facilitates TrkB signaling and function in rat hippocampal neurons. Front Cell Neurosci 13:485
Barbacid M (1995) Structural and functional properties of the TRK family of neurotrophin receptors. Ann N Y Acad Sci 766:442–458
Skaper SD (2012) The neurotrophin family of neurotrophic factors: an overview. Methods Mol Biol 486:1–12
Meeker RB, Williams KS (2015) The p75 neurotrophin receptor: at the crossroad of neural repair and death. Neural Regen Res 10:721–725
Shi J, Longo FM, Massa SM (2013) A small molecule p75NTR ligand protects neurogenesis after traumatic brain injury. Stem cells 31:2561–2574
Scardigli R, Capelli P, Vignone D, Brandi R, Ceci M, Regina F, Piras E, Cintoli S et al (2014) Neutralization of nerve growth factor impairs proliferation and differentiation of adult neural progenitors in the subventricular zone. Stem Cells 32:2516–2528
Numakawa T, Richards M, Adachi N, Kishi S, Kunugi H, Hashido K (2011) MicroRNA function and neurotrophin BDNF. Neurochem Int 59:551–558
Imam JS, Plyler JR, Bansal H, Prajapati S, Bansal S, Rebeles J, Chen HI, Chang YF et al (2012) Genomic loss of tumor suppressor miRNA-204 promotes cancer cell migration and invasion by activating AKT/mTOR/Rac1 signaling and actin reorganization. PLoS One 7:e52397
Xiao J, Wang Y, Bellusci S, Li X (2015) Pharmacological application of growth factors: basic and clinical. Biomed Res Int 141794
Gascon S, Jann J, Langlois-Blais C, Plourde M, Lavoie C, Faucheux N (2021) Peptides Derived from Growth Factors to Treat Alzheimer’s Disease. Int J Mol Sci 22:6071
Castrén E (2004) Neurotrophins as mediators of drug effects on mood, addiction, and neuroprotection. Mol Neurobiol 29:289–301
Shi J (2015) Regulatory networks between neurotrophins and miRNAs in brain diseases and cancers. Acta Pharmacol Sin 36:149–157
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297
O’Brien J, Hayder H, Zayed Y, Peng C (2018) Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front Endocrinol 9:402
Wahid F, Shehzad A, Khan T, Kim YY (2010) MicroRNAs: synthesis, mechanism, function, and recent clinical trials. Biochim Biophys Acta 1803:1231–1243
Olejniczak M, Kotowska-Zimmer A, Krzyzosiak W (2018) Stress-induced changes in miRNA biogenesis and functioning. Cell Mol Life Sci 75:177–191
Sheu-Gruttadauria J, MacRae IJ (2017) Structural foundations of RNA silencing by Argonaute. J Mol Biol 429:2619–2639
Huang S, He X (2010) microRNAs: tiny RNA molecules, huge driving forces to move the cell. Protein Cell 1:916–926
Ambros V (2004) The functions of animal microRNAs. Nature 431:350–355
Nguyen LS, Fregeac J, Bole-Feysot C, Cagnard N, Iyer A, Anink J, Aronica E, Alibeu O et al (2018) Role of miR-146a in neural stem cell differentiation and neural lineage determination: relevance for neurodevelopmental disorders. Mol Autism 9:38
Rajgor D (2018) Macro roles for microRNAs in neurodegenerative diseases. Noncoding RNA Res 3:154–159
Vo N, Klein ME, Varlamova O, Keller DM, Yamamoto T, Goodman RH, Impey S (2005) A cAMP-response element binding protein-induced microRNA regulates neuronal morphogenesis. Proc Natl Acad Sci U S A 102:16426–16431
Thomas KT, Gross C, Bassell GJ (2018) MicroRNAs sculpt neuronal communication in a tight balance that is lost in neurological disease. Front Mol Neurosci 11:455
Eacker SM, Dawson TM, Dawson VL (2009) Understanding microRNAs in neurodegeneration. Nat Rev Neurosci 10:837–841
Galloway DA, Moore CS (2016) miRNAs as emerging regulators of oligodendrocyte development and differentiation. Front Cell Dev Biol 17(4):59
Im HI, Kenny PJ (2012) MicroRNAs in neuronal function and dysfunction. Trends Neurosci 35:325–334
Bai Y, Su X, Piao L, Jin Z, Jin R (2021) Involvement of astrocytes and microRNA dysregulation in neurodegenerative diseases: from pathogenesis to therapeutic potential. Front Mol Neurosci 14:556215
Guo Y, Hong W, Wang X, Zhang P, Körner H, Tu J, Wei W (2019) MicroRNAs in microglia: how do MicroRNAs affect activation, inflammation, polarization of microglia and mediate the interaction between microglia and glioma? Front Mol Neurosci 12:125
Eom TY, Han SB, Kim J, Blundon JA, Wang YD, Yu J, Anderson K, Kaminski DB et al (2020) Schizophrenia-related microdeletion causes defective ciliary motility and brain ventricle enlargement via microRNA-dependent mechanisms in mice. Nat Commun 11:912
Maciotta Rolandin S, Meregalli M, Torrente Y (2013) The involvement of microRNAs in neurodegenerative diseases. Front Cell Neurosci 7:265
Zhao Y, Bhattacharjee S, Jones BM, Hill J, Dua P, Lukiw WJ (2014) Regulation of neurotropic signaling by the inducible, NF-kB-sensitive miRNA-125b in Alzheimer’s disease (AD) and in primary human neuronal-glial (HNG) cells. Mol Neurobiol 50:97–106
Bahlakeh G, Gorji A, Soltani H, Ghadiri T (2021) MicroRNA alterations in neuropathologic cognitive disorders with an emphasis on dementia: lessons from animal models. J Cell Physiol 236:806–823
Gao J, Wang WY, Mao YW, Gräff J, Guan JS, Pan L, Mak G, Kim D et al (2010) A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature 466:1105–1109
Colucci-D’Amato L, Speranza L, Volpicelli F (2020) Neurotrophic factor BDNF, physiological functions and therapeutic potential in depression, neurodegeneration and brain cancer. Int J Mol Sci 21:7777
Shmakova AA, Rysenkova KD, Ivashkina OI, Gruzdeva AM, Klimovich PS, Popov VS, Rubina KA, Anokhin KV et al (2021) Early induction of neurotrophin receptor and miRNA genes in mouse brain after pentilenetetrazole-induced neuronal activity. Biochemistry (Mosc) 86:1326–1341
Martinez NJ, Walhout AJ (2009) The interplay between transcription factors and microRNAs in genome-scale regulatory networks. BioEssays 31:435–445
Keifer J, Zheng Z, Ambigapathy G (2015) A microRNA-BDNF negative feedback signaling loop in brain: implications for Alzheimer’s disease. Microrna 4:101–108
Konovalova J, Gerasymchuk D, Arroyo SN, Kluske S, Mastroianni F, Pereyra AV, Domanskyi A (2021) Human-specific regulation of neurotrophic factors MANF and CDNF by microRNAs. Int J Mol Sci 22:9691
Huang EJ, Reichardt LF (2001) Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci 24:677–736
Condrat CE, Thompson DC, Barbu MG, Bugnar OL, Boboc A, Cretoiu D, Suciu N, Cretoiu SM, Voinea SC (2020) miRNAs as biomarkers in disease: latest findings regarding their role in diagnosis and prognosis. Cells 9:276
Guidi M, Muiños-Gimeno M, Kagerbauer B, Martí E, Estivill X, Espinosa-Parrilla Y (2010) Overexpression of miR-128 specifically inhibits the truncated isoform of NTRK3 and upregulates BCL2 in SH-SY5Y neuroblastoma cells. BMC Mol Biol 11:1–7
Lu B, Pang PT, Woo NH (2005) The yin and yang of neurotrophin action. Nat Rev Neurosci 6:603–614
Barbacid M (1994) The Trk family of neurotrophin receptors. J Neurobiol 25:1386–1403
Vislovukh A, Vargas TR, Polesskaya A, Groisman I (2014) Role of 3’-untranslated region translational control in cancer development, diagnostics and treatment. World J Biol Chem 5:40–57
Agarwal V, Bell GW, Nam JW, Bartel DP (2015) Predicting effective microRNA target sites in mammalian mRNAs. Elife 4:e05005
Hong EJ, McCord AE, Greenberg ME (2008) A biological function for the neuronal activity-dependent component of Bdnf transcription in the development of cortical inhibition. Neuron 60:610–624
Laneve P, Di Marcotullio L, Gioia U, Fiori ME, Ferretti E, Gulino A, Bozzoni I, Caffarelli E (2007) The interplay between microRNAs and the neurotrophin receptor tropomyosin-related kinase C controls proliferation of human neuroblastoma cells. Proc Natl Acad Sci U S A 104:7957–7962
Wang T, Liu YY, Wang X, Yang N, Zhu HB, Zuo PP (2010) Protective effects of octacosanol on 6-hydroxydopamine-induced Parkinsonism in rats via regulation of ProNGF and NGF signaling. Acta Pharmacol Sin 31:765–774
Jauhari A, Singh T, Singh P, Parmar D, Yadav S (2018) Regulation of miR-34 Family in Neuronal Development. Mol Neurobiol 55:936–945
Pandey A, Singh P, Jauhari A, Singh T, Khan F, Pant AB, Parmar D, Yadav S (2015) Critical role of the miR-200 family in regulating differentiation and proliferation of neurons. J Neurochem 133:640–652
Zou H, Ding Y, Shi W, Xu X, Gong A, Zhang Z, Liu J (2015) MicroRNA-29c/PTEN pathway is involved in mice brain development and modulates neurite outgrowth in PC12 cells. Cell Mol Neurobiol 35:313–322
Ikenaka K, Nakahira K, Takayama C, Wada K, Hatanaka H, Mikoshiba K (1990) Nerve growth factor rapidly induces expression of the 68-kDa neurofilament gene by posttranscriptional modification in PC12h-R cells. J Biol Chem 265:19782–19785
de Cubas AA, Leandro-García LJ, Schiavi F, Mancikova V, Comino-Méndez I, Inglada-Pérez L, Perez-Martinez M, Ibarz N et al (2013) Integrative analysis of miRNA and mRNA expression profiles in pheochromocytoma and paraganglioma identifies genotype-specific markers and potentially regulated pathways. Endocr Relat Cancer 20:477–493
Hamada N, Fujita Y, Kojima T, Kitamoto A, Akao Y, Nozawa Y, Ito M (2012) MicroRNA expression profiling of NGF-treated PC12 cells revealed a critical role for miR-221 in neuronal differentiation. Neurochem Int 60:743–750
Montalban E, Mattugini N, Ciarapica R, Provenzano C, Savino M, Scagnoli F, Prosperini G, Carissimi C et al (2014) MiR-21 is an Ngf-modulated microRNA that supports Ngf signaling and regulates neuronal degeneration in PC12 cells. Neuromolecular Med 16:415–430
Hu J, Huang CX, Rao PP, Zhou JP, Wang X, Tang L, Liu MX, Zhang GG (2019) Inhibition of microRNA-155 attenuates sympathetic neural remodeling following myocardial infarction via reducing M1 macrophage polarization and inflammatory responses in mice. Eur J Pharmacol 851:122–132
Huang J, Zhao L, Fan Y, Liao L, Ma PX, Xiao G, Chen D (2019) The microRNAs miR-204 and miR-211 maintain joint homeostasis and protect against osteoarthritis progression. Nat Commun 10:2876
Ong J, Timens W, Rajendran V, Algra A, Spira A, Lenburg ME, Campbell JD, van den Berge M et al (2017) Identification of transforming growth factor-beta-regulated microRNAs and the microRNA-targetomes in primary lung fibroblasts. PLoS One 12:e0183815
Liu H, Tan N, Xu D, Li CY, Xian GJ (2020) NGF and CNTF expression and regulation mechanism by miRNA in acute paralytic strabismus. Int Ophthalmol 40:975–984
Yang S, Tang W, He Y, Wen L, Sun B, Li S (2018) Long non-coding RNA and microRNA-675/let-7a mediates the protective effect of melatonin against early brain injury after subarachnoid hemorrhage via targeting TP53 and neural growth factor. Cell Death Dis 9:99
Wu N, Meng F, Invernizzi P, Bernuzzi F, Venter J, Standeford H, Onori P, Marzioni M et al (2016) The secretin/secretin receptor axis modulates liver fibrosis through changes in transforming growth factor-β1 biliary secretion in mice. Hepatology 64:865–879
Li S, Wang X, Gu Y, Chen C, Wang Y, Liu J, Hu W, Yu B et al (2015) Let-7 microRNAs regenerate peripheral nerve regeneration by targeting nerve growth factor. Mol Ther 23:423–433
Asahchop EL, Branton WG, Krishnan A, Chen PA, Yang D, Kong L, Zochodne DW, Brew BJ, Gill MJ (2018) Power, C. HIV-associated sensory polyneuropathy and neuronal injury are associated with miRNA-455–3p induction. JCI Insight 3
Glaser S, Meng F, Han Y, Onori P, Chow BK, Francis H, Venter J, McDaniel K et al (2014) Secretin stimulates biliary cell proliferation by regulating expression of microRNA 125b and microRNA let7a in mice. Gastroenterol 146:1795–808.e12
Yuan H, Du S, Chen L, Xu X, Wang Y, Ji F (2020) Hypomethylation of nerve growth factor (NGF) promotes binding of C/EBPα and contributes to inflammatory hyperalgesia in rats. J Neuroinflammation 17:34
Goswami S, Banerjee A, Kumari B, Bandopadhyay B, Bhattacharya N, Basu N, Vrati S, Banerjee A (2017) Differential expression and significance of circulating microRNAs in cerebrospinal fluid of acute encephalitis patients infected with Japanese Encephalitis virus. Mol Neurobiol 54:1541–1551
Piao JM, Wu W, Yang ZX, Li YZ, Luo Q, Yu JL (2018) MicroRNA-381 Favors Repair of Nerve Injury Through Regulation of the SDF-1/CXCR4 Signaling pathway via LRRC4 in acute cerebral ischemia after cerebral lymphatic blockage. Cell Physiol Biochem 46:890–906
Radzikinas K, Aven L, Jiang Z, Tran T, Paez-Cortez J, Boppidi K, Lu J, Fine A et al (2011) A Shh/miR-206/BDNF cascade coordinates innervation and formation of airway smooth muscle. J Neurosci 31:15407–15415
Caputo V, Sinibaldi L, Fiorentino A, Parisi C, Catalanotto C, Pasini A, Cogoni C, Pizzuti A (2011) Brain derived neurotrophic factor (BDNF) expression is regulated by microRNAs miR-26a and miR-26b allele-specific binding. PLoS One 6:e28656
Jiang Y, Zhu J (2015) Effects of sleep deprivation on behaviors and abnormal hippocampal BDNF/miR-10B expression in rats with chronic stress depression. Int J Clin Exp Pathol 8:586–593
Croce N, Gelfo F, Ciotti MT, Federici G, Caltagirone C, Bernardini S, Angelucci F (2013) NPY modulates miR-30a-5p and BDNF in opposite direction in an in vitro model of Alzheimer disease: a possible role in neuroprotection? Mol Cell Biochem 376:189–195
Hu JJ, Qin LJ, Liu ZY, Liu P, Wei HP, Wang HY, Zhao CC, Ge ZM (2020) miR-15a regulates oxygen glucose deprivation/reperfusion (OGD/R)-induced neuronal injury by targeting BDNF. Kaohsiung J Med Sci 36:27–34
You YH, Qin ZQ, Zhang HL, Yuan ZH, Yu X (2019) MicroRNA-153 promotes brain-derived neurotrophic factor and hippocampal neuron proliferation to alleviate autism symptoms through inhibition of JAK-STAT pathway by LEPR. Biosci Rep 39
Wu BW, Wu MS, Guo JD (2018) Effects of microRNA-10a on synapse remodeling in hippocampal neurons and neuronal cell proliferation and apoptosis through the BDNF-TrkB signaling pathway in a rat model of Alzheimer’s disease. J Cell Physiol 233:5281–5292
Zhang K, Wu S, Li Z, Zhou J (2017) MicroRNA-211/BDNF axis regulates LPS-induced proliferation of normal human astrocyte through PI3K/AKT pathway. Biosci Rep 37 BSR20170755
Liyanage VR, Rastegar M (2014) Rett syndrome and MeCP2. Neuromolecular med 16:231–264
Wang H, Chan SA, Ogier M, Hellard D, Wang Q, Smith C, Katz DM (2006) Dysregulation of brain-derived neurotrophic factor expression and neurosecretory function in Mecp2 null mice. JNeurosci 26:10911–10915
Downs J, Rodger J, Li C, Tan X, Hu N, Wong K, De Klerk N, Leonard H (2018) Environmental enrichment intervention for Rett syndrome: an individually randomised stepped wedge trial. rphanet. J. Rare Dis 13:1–9
Chang Q, Khare G, Dani V, Nelson S, Jaenisch R (2006) The disease progression of Mecp2 mutant mice is affected by the level of BDNF expression. Neuron 49:341–348
Matijevic T, Knezevic J, Slavica M, Pavelic J (2009) Rett syndrome: from the gene to the disease. Eur Neurol 61:3–10
Gao Y, Su J, Guo W, Polich ED, Magyar DP, Xing Y, Li H, Smrt RD, Chang Q, Zhao X (2015) Inhibition of miR-15a promotes BDNF expression and rescues dendritic maturation deficits in MeCP2-deficient neurons. Stem Cells 33:1618–1629
Wang J, Huang Q, Ding J, Wang X (2019) Elevated serum levels of brain-derived neurotrophic factor and miR-124 in acute ischemic stroke patients and the molecular mechanism. 3 Biotech 9:386
Huang SW, Ali ND, Zhong L, Shi J (2018) MicroRNAs as biomarkers for human glioblastoma: progress and potential. Acta Pharmacol Sin 39:1405–1413
Wang L, Liu Y, Song J (2018) MicroRNA-103 suppresses glioma cell proliferation and invasion by targeting the brain-derived neurotrophic factor. Mol Med Rep 17:4083–4089
Li EY, Zhao PJ, Jian J, Yin BQ, Sun ZY, Xu CX, Tang YC, Wu H (2019) Vitamin B1 and B12 mitigates neuron apoptosis in cerebral palsy by augmenting BDNF expression through MALAT1/miR-1 axis. Cell Cycle 18:2849–2859
Huang W, Liu X, Cao J, Meng F, Li M, Chen B, Zhang J (2015) miR-134 regulates ischemia/reperfusion injury-induced neuronal cell death by regulating CREB signaling. J Mol Neurosci 55:821–829
Jiangpan P, Qingsheng M, Zhiwen Y, Tao Z (2016) Emerging Role of microRNA in Neuropathic Pain. Curr Drug Metab 17:336–344
Tan M, Shen L, Hou Y (2020) Epigenetic modification of BDNF mediates neuropathic pain via miR-30a-3p/EP300 axis in CCI rats. Biosci Rep 40
Zhao X, Shu F, Wang X, Wang F, Wu L, Li L, Lv H (2019) Inhibition of microRNA-375 ameliorated ketamine-induced neurotoxicity in human embryonic stem cell derived neurons. Eur J Pharmacol 844:56–64
Jiang JD, Zheng XC, Huang FY, Gao F, You MZ, Zheng T (2019) MicroRNA-107 regulates anesthesia-induced neural injury in embryonic stem cell derived neurons. IUBMB Life 71:20–27
Xie W, Yang SY, Zhang Q, Zhou Y, Wang Y, Liu R, Wang W, Shi J et al (2018) Knockdown of microRNA-21 promotes neurological recovery after acute spinal cord injury. Neurochem Res 43:1641–1649
Shen J, Xu L, Qu C, Sun H, Zhang J (2018) Resveratrol prevents cognitive deficits induced by chronic unpredictable mild stress: Sirt1/miR-134 signalling pathway regulates CREB/BDNF expression in hippocampus in vivo and in vitro. Behav Brain Res 349:1–7
Ureña-Peralta JR, Alfonso-Loeches S, Cuesta-Diaz CM, García-García F, Guerri C (2018) Deep sequencing and miRNA profiles in alcohol-induced neuroinflammation and the TLR4 response in mice cerebral cortex. Sci Rep 8:15913
Li Z, Yu Y, Kang J, Zheng Y, Xu J, Xu K, Hou K, Hou Y et al (2020) MicroRNA-124 overexpression in schwann cells promotes schwann cell-astrocyte integration and inhibits glial scar formation ability. Front Cell Neurosci 14:144
McCreary JK, Erickson ZT, Hao Y, Ilnytskyy Y, Kovalchuk I, Metz GA (2016) Environmental intervention as a therapy for adverse programming by ancestral stress. Sci Rep 6:37814
Zhang M, Zhu Y, Wei M, Liu H (2020) Neuroprotective effects of miR-30c on rats with cerebral ischemia/reperfusion injury by targeting SOX9. Pathol Res Pract 216:153271
Howe EN, Cochrane DR, Cittelly DM, Richer JK (2012) miR-200c targets a NF-κB up-regulated TrkB/NTF3 autocrine signaling loop to enhance anoikis sensitivity in triple negative breast cancer. PLoS One 7:e49987
Liu D, Song L, Dai Z, Guan H, Kang H, Zhang Y, Yan W, Zhao X et al (2018) MiR-429 suppresses neurotrophin-3 to alleviate perineural invasion of pancreatic cancer. Biochem Biophys Res Commun 505:1077–1083
Wong J (2014) Regulation of a TrkB Alternative Transcript by microRNAs. Dement Geriatr Cogn Dis Extra 4:364–374
Liao W, Zhang H, Feng C, Wang T, Zhang Y, Tang S (2014) Downregulation of TrkA protein expression by miRNA 92a promotes the proliferation and migration of human neuroblastoma cells. Mol Med Rep 10:778–784
Zhao H, Li Y, Chen L, Shen C, Xiao Z, Xu R, Wang J, Luo Y (2019) HucMSCs-Derived miR-206-knockdown exosomes contribute to neuroprotection in subarachnoid hemorrhage induced early brain injury by targeting BDNF. Neuroscience 417:11–23
Forstner AJ, Basmanav FB, Mattheisen M, Böhmer AC, Hollegaard MV, Janson E, Strengman E, Priebe L et al (2014) Investigation of the involvement of MIR185 and its target genes in the development of schizophrenia. J Psychiatry Neurosci 39:386–396
Irmady K, Jackman KA, Padow VA, Shahani N, Martin LA, Cerchietti L, Unsicker K, Iadecola C et al (2014) Mir-592 regulates the induction and cell death-promoting activity of p75NTR in neuronal ischemic injury. J Neurosci 34:3419–3428
Dzieran J, Rodriguez Garcia A, Westermark UK, Henley AB, Eyre Sánchez E, Träger C, Johansson HJ, Lehtiö J et al (2018) MYCN-amplified neuroblastoma maintains an aggressive and undifferentiated phenotype by deregulation of estrogen and NGF signaling. Proc Natl Acad Sci U S A 115:E1229–E1238
Wen Y, Liu G, Jia L, Ji W, Li H (2019) MicroRNA-141 binds to the nerve growth factor receptor associated protein 1 gene and restores the erectile function of diabetic rats through down-regulating the nerve growth factor/neurotrophin receptor p75 (NGF/p75NTR) signaling. J Cell Biochem 120:7940–7951
Remenyi J, Hunter CJ, Cole C, Ando H, Impey S, Monk CE, Martin KJ, Barton GJ et al (2010) Regulation of the miR-212/132 locus by MSK1 and CREB in response to neurotrophins. Biochem J 4(28):281–291
Terasawa K, Ichimura A, Sato F, Shimizu K, Tsujimoto G (2009) Sustained activation of ERK1/2 by NGF induces microRNA-221 and 222 in PC12 cells. Febs j 276:3269–3276
Mullenbrock S, Shah J, Cooper GM (2011) Global expression analysis identified a preferentially nerve growth factor-induced transcriptional program regulated by sustained mitogen-activated protein kinase/extracellular signal-regulated kinase (ERK) and AP-1 protein activation during PC12 cell differentiation. J Biol Chem 286:45131–45145
Zhang J, Zhang J, Liu LH, Zhou Y, Li YP, Shao ZH, Wu YJ, Li MJ, Fan YY et al (2011) Effects of miR-541 on neurite outgrowth during neuronal differentiation. Cell Biochem Funct 29:279–286
Pokharel S, Lee CH, Gilyazova N, Ibeanu GC (2018) Analysis of Gene Expression and Neuronal Phenotype in Neuroscreen-1 (NS-1) Cells. Int J Biomed Investig 1:115
Kashyap M, Pore S, Chancellor M, Yoshimura N, Tyagi P (2016) Bladder overactivity involves overexpression of MicroRNA 132 and nerve growth factor. Life Sci 167:98–104
Wu D, Qian T, Hong J, Li G, Shi W, Xu J (2017) MicroRNA 494 inhibits nerve growth factor induced cell proliferation by targeting cyclin D1 in human corneal epithelial cells. Mol Med Rep 16:4133–4142
Retamales-Ortega R, Oróstica L, Vera C, Cuevas P, Hernández A, Hurtado I, Vega M, Romero C (2017) Role of nerve growth factor (NGF) and miRNAs in epithelial ovarian cancer. Int J Mol Sci 18:507
Wang B, Pan L, Wei M, Wang Q, Liu WW, Wang N, Jiang XY, Zhang X et al (2015) FMRP-mediated axonal delivery of miR-181d regulates axon elongation by locally targeting Map1b and Calm1. Cell Rep 13:2794–2807
Zhang L, Li X, Shen Y, Lin X, Wu M (2019) Transdifferentiation effects and related mechanisms of nerve growth factor and internal limiting membrane on Müller cells. Exp Eye Res 180:146–154
Chatterjee N, Sanphui P, Kemeny S, Greene LA, Biswas SC (2016) Role and regulation of Cdc25A phosphatase in neuron death induced by NGF deprivation or β-amyloid. Cell Death Discov 2:16083
Neumann E, Brandenburger T, Santana-Varela S, Deenen R, Köhrer K, Bauer I, Hermanns H, Wood JN et al (2016) MicroRNA-1-associated effects of neuron-specific brain-derived neurotrophic factor gene deletion in dorsal root ganglia. Mol Cell Neurosci 75:36–43
Jauhari A, Singh T, Yadav S (2018) Expression of miR-145 and its target proteins are regulated by miR-29b in differentiated neurons. Mol Neurobiol 55:8978–8990
Schratt GM, Tuebing F, Nigh EA, Kane CG, Sabatini ME, Kiebler M, Greenberg ME (2006) A brain-specific microRNA regulates dendritic spine development. Nature 439:283–289
Le MT, Xie H, Zhou B, Chia PH, Rizk P, Um M, Udolph G, Yang H et al (2009) MicroRNA-125b promotes neuronal differentiation in human cells by repressing multiple targets. Mol Cell Biol 29:5290–5305
Kawashima H, Numakawa T, Kumamaru E, Adachi N, Mizuno H, Ninomiya M, Kunugi H, Hashido K (2010) Glucocorticoid attenuates brain-derived neurotrophic factor-dependent upregulation of glutamate receptors via the suppression of microRNA-132 expression. Neuroscience 165:1301–1311
Tu Z, Li Y, Dai Y, Li L, Lv G, Chen I, Wang B (2017) MiR-140/BDNF axis regulates normal human astrocyte proliferation and LPS-induced IL-6 and TNF-α secretion. Biomed Pharmacother 91:899–905
Sun YX, Yang J, Wang PY, Li YJ, Xie SY, Sun RP (2013) Cisplatin regulates SH-SY5Y cell growth through downregulation of BDNF via miR-16. Oncol Rep 30:2343–2349
Cui M, Xiao H, Li Y, Dong J, Luo D, Li H, Feng G, Wang H et al (2017) Total abdominal irradiation exposure impairs cognitive function involving miR-34a-5p/BDNF axis. Biochim Biophys Acta Mol Basis Dis 1863:2333–2341
Huang W, Meng F, Cao J, Liu X, Zhang J, Li M (2017) Neuroprotective role of exogenous brain-derived neurotrophic factor in hypoxia-hypoglycemia-induced hippocampal neuron injury via regulating Trkb/MiR134 signaling. J Mol Neurosci 62:35–42
Eyileten C, Sharif L, Wicik Z, Jakubik D, Jarosz-Popek J, Soplinska A, Postula M, Czlonkowska A et al (2021) The relation of the brain-derived neurotrophic factor with MicroRNAs in neurodegenerative diseases and ischemic stroke. Mol Neurobiol 58:329–347
Miranda M, Morici JF, Zanoni MB, Bekinschtein P (2019) Brain-derived neurotrophic factor: a key molecule for memory in the healthy and the pathological brain. Front Cell Neurosci 13:363
Muiños-Gimeno M, Espinosa-Parrilla Y, Guidi M, Kagerbauer B, Sipilä T, Maron E, Pettai K, Kananen L et al (2011) Human microRNAs miR-22, miR-138-2, miR-148a, and miR-488 are associated with panic disorder and regulate several anxiety candidate genes and related pathways. Biol Psychiatry 69:526–533
Piotrzkowska D, Miller E, Kucharska E, Niwald M, Majsterek I (2021) Association of miRNA and mRNA levels of the clinical onset of multiple sclerosis patients. Biology (Basel) 10:554
Brennan GP, Henshall DC (2020) MicroRNAs as regulators of brain function and targets for treatment of epilepsy. Nat Rev Neurol 16:506–519
Banerjee PN, Filippi D, Allen Hauser W (2009) The descriptive epidemiology of epilepsy-a review. Epilepsy Res 85:31–45
Lauterborn JC, Poulsen FR, Stinis CT, Isackson PJ, Gall CM (1998) Transcript-specific effects of adrenalectomy on seizure-induced BDNF expression in rat hippocampus. Brain Res Mol Brain Res 55:81–91
Zhang L, Fan D, Wang Q, Baier G (2018) Effects of brain-derived neurotrophic factor and noise on transitions in temporal lobe epilepsy in a hippocampal network. Chaos 28:106322
Heinrich C, Lähteinen S, Suzuki F, Anne-Marie L, Huber S, Häussler U, Haas C, Larmet Y et al (2011) Increase in BDNF-mediated TrkB signaling promotes epileptogenesis in a mouse model of mesial temporal lobe epilepsy. Neurobiol Dis 42:35–47
Duan W, Chen Y, Wang XR (2018) MicroRNA 155 contributes to the occurrence of epilepsy through the PI3K/Akt/mTOR signaling pathway. Int J Mol Med 42:1577–1584
Cai Z, Li S, Li S, Song F, Zhang Z, Qi G, Li T, Qiu J et al (2016) Antagonist Targeting microRNA-155 Protects against lithium-pilocarpine-induced status epilepticus in C57BL/6 mice by activating brain-derived neurotrophic factor. Front Pharmacol 7:129
Xiang L, Ren Y, Cai H, Zhao W, Song Y (2015) MicroRNA-132 aggravates epileptiform discharges via suppression of BDNF/TrkB signaling in cultured hippocampal neurons. Brain Res 1622:484–495
Zheng P, Bin H, Chen W (2019) Inhibition of microRNA-103a inhibits the activation of astrocytes in hippocampus tissues and improves the pathological injury of neurons of epilepsy rats by regulating BDNF. Cancer Cell Int 19:109
Peng J, Omran A, Ashhab MU, Kong H, Gan N, He F, Yin F (2013) Expression patterns of miR-124, miR-134, miR-132, and miR-21 in an immature rat model and children with mesial temporal lobe epilepsy. J Mol Neurosci 50:291–297
Risbud RM, Porter BE (2013) Changes in microRNA expression in the whole hippocampus and hippocampal synaptoneurosome fraction following pilocarpine induced status epilepticus. PLoS One 8:e53464
Chak K, Roy-Chaudhuri B, Kim HK, Kemp KC, Porter BE, Kay MA (2016) Increased precursor microRNA-21 following status epilepticus can compete with mature microRNA-21 to alter translation. Exp Neurol 286:137–146
Risbud RM, Lee C, Porter BE (2011) Neurotrophin-3 mRNA a putative target of miR21 following status epilepticus. Brain Res 1424:53–59
de Faria O, Moore CS Jr, Kennedy TE, Antel JP, Bar-ORA Dhaunchak AS (2012) MicroRNA dysregulation in multiple sclerosis. Front Genet 3:311
Ksiazek-Winiarek D, Szpakowski P, Turniak M, Szemraj J, Glabinski A (2017) IL-17 exerts anti-apoptotic effect via miR-155-5p downregulation in experimental autoimmune encephalomyelitis. J Mol Neurosci 63:320–332
Pons V, Rivest S (2020) Beneficial roles of microglia and growth factors in MS, a brief review. Front Cell Neurosci 14:284
Kalinowska-Lyszczarz A, Losy J (2012) The role of neurotrophins in multiple sclerosis-pathological and clinical implications. Int J Mol Sci 13:13713–13725
Acosta CM, Cortes C, MacPhee H, Namaka MP (2013) Exploring the role of nerve growth factor in multiple sclerosis: implications in myelin repair. CNS Neurol Disord Drug Targets 12:1242–1256
Venkatesha SH, Dudics S, Song Y, Mahurkar A, Moudgil KD (2018) The miRNA expression profile of experimental autoimmune encephalomyelitis reveals novel potential disease biomarkers. Int J Mol Sci 19:3990
Razavi S, Nazem G, Mardani M, Esfandiari E, Salehi H, Esfahani SH (2015) Neurotrophic factors and their effects in the treatment of multiple sclerosis. Adv Biomed Res 4:53
Luo C, Jian C, Liao Y, Huang Q, Wu Y, Liu X, Zou D, Wu Y (2017) The role of microglia in multiple sclerosis. Neuropsychiatr Dis Treat 13:1661–1667
Ponomarev ED, Veremeyko T, Barteneva N, Krichevsky AM, Weiner HL (2011) MicroRNA-124 promotes microglia quiescence and suppresses EAE by deactivating macrophages via the C/EBP-α-PU 1 pathway. Nat Med 17:64–70
Gupta N, Jadhav S, Tan KL, Saw G, Mallilankaraman KB, Dheen ST (2020) miR-142-3p Regulates BDNF expression in activated rodent microglia through its target CAMK2A. Front Cell Neurosci 14:132
Konovalova J, Gerasymchuk D, Parkkinen I, Chmielarz P, Domanskyi A (2019) Interplay between MicroRNAs and oxidative stress in neurodegenerative diseases. Int J Mol Sci 20
Giampà C, Montagna E, Dato C, Melone MA, Bernardi G, Fusco FR (2013) Systemic delivery of recombinant brain derived neurotrophic factor (BDNF) in the R6/2 mouse model of Huntington’s disease. PLoS One 8:e64037
Zuccato C, Ciammola A, Rigamonti D, Leavitt BR, Goffredo D, Conti L, MacDonald ME, Friedlander RM et al (2001) Loss of huntingtin-mediated BDNF gene transcription in Huntington’s disease. Science 293:493–498
Yu C, Li CH, Chen S, Yoo H, Qin X, Park H (2018) Decreased BDNF Release in Cortical Neurons of a Knock-in Mouse Model of Huntington’s Disease. Sci Rep 8:16976
Bithell A, Johnson R, Buckley NJ (2009) Transcriptional dysregulation of coding and non-coding genes in cellular models of Huntington’s disease. Biochem Soc Trans 37:1270–1275
Liu T, Im W, Mook-Jung I, Kim M (2015) MicroRNA-124 slows down the progression of Huntington’s disease by promoting neurogenesis in the striatum. Neural Regen Res 10:786–791
Müller S (2014) In silico analysis of regulatory networks underlines the role of miR-10b-5p and its target BDNF in Huntington’s disease. Transl Neurodegener 3:17
Tung CW, Huang PY, Chan SC, Cheng PH, Yang SH (2021) The regulatory roles of microRNAs toward pathogenesis and treatments in Huntington’s disease. J Biomed Sci 28:59
Domanskyi A, Saarma M, Airavaara M (2015) Prospects of Neurotrophic Factors for Parkinson’s Disease: Comparison of Protein and Gene Therapy. Hum Gene Ther 26:550–559
Ghose J, Sinha M, Das E, Jana NR, Bhattacharyya NP (2011) Regulation of miR-146a by RelA/NFkB and p53 in STHdh(Q111)/Hdh(Q111) cells, a cell model of Huntington’s disease. PLoS One 6:e23837
Giuffrida ML, Copani A, Rizzarelli E (2018) A promising connection between BDNF and Alzheimer’s disease. Aging (Albany NY) 10:1791–1792
Mitra S, Behbahani H, Eriksdotter M (2019) Innovative Therapy for Alzheimer’s Disease-With Focus on Biodelivery of NGF. Front Neurosci 13:38
Mellios N, Huang HS, Grigorenko A, Rogaev E, Akbarian S (2008) A set of differentially expressed miRNAs, including miR-30a-5p, act as post-transcriptional inhibitors of BDNF in prefrontal cortex. Hum Mol Genet 17:3030–3042
Qiu L, Tan EK, Zeng L (2015) microRNAs and neurodegenerative diseases. Adv Exp Med Biol 888:85–105
Li W, Li X, Xin X, Kan PC, Yan Y (2016) MicroRNA-613 regulates the expression of brain-derived neurotrophic factor in Alzheimer’s disease. Biosci Trends 10:372–377
Croce N, Gelfo F, Ciotti MT, Federici G, Caltagirone C, Bernardini S, Angelucci F (2013) NPY modulates miR-30a-5p and BDNF in opposite direction in an in vitro model of Alzheimer disease: a possible role in neuroprotection? Mol Cell Biochem 376:189–195
Yang G, Song Y, Zhou X, Deng Y, Liu T, Weng G, Yu D, Pan S (2015) DNA methyltransferase 3, a target of microRNA-29c, contributes to neuronal proliferation by regulating the expression of brain-derived neurotrophic factor. Mol Med Rep 12:1435–1442
Li C, Wu X, Liu S, Zhao Y, Zhu J, Liu K (2019) Roles of neuropeptide Y in neurodegenerative and neuroimmune diseases. Front Neurosci 13:869
Thomas KT, Gross C, Bassell GJ (2018) microRNAs sculpt neuronal communication in a tight balance that is lost in neurological disease. Front Mol Neurosci 11:455
Zhang J, Liu Z, Pei Y, Yang W, Xie C, Long S (2018) MicroRNA-322 cluster promotes tau phosphorylation via targeting brain-derived neurotrophic factor. Neurochem Res 43:736–744
Shu B, Zhang X, Du G, Fu Q, Huang L (2018) MicroRNA-107 prevents amyloid-β-induced neurotoxicity and memory impairment in mice. Int J Mol Med 41:1665–1672
Kumar S, Reddy PH (2018) MicroRNA-455-3p as a potential biomarker for Alzheimer’s disease: an update. Front Aging Neurosci 10:41
Wu BW, Wu MS, Guo JD (2018) Effects of microRNA-10a on synapse remodeling in hippocampal neurons and neuronal cell proliferation and apoptosis through the BDNF-TrkB signaling pathway in a rat model of Alzheimer’s disease. J Cell Physiol 233:5281–5292
Heman-Ackah SM, Hallegger M, Rao MS, Wood MJ (2013) RISC in PD: the impact of microRNAs in Parkinson’s disease cellular and molecular pathogenesis. Front Mol Neurosci 6:40
Chmielarz P, Konovalova J, Najam SS, Alter H, Piepponen TP, Erfle H, Sonntag KC, Schütz G et al (2017) Dicer and microRNAs protect adult dopamine neurons. Cell Death Dis 8:e2813
Bhardwaj R, Deshmukh R (2018) Neurotrophic factors and Parkinson’s disease. Clin Investig 7:53–62
Ferreira RN, de Miranda AS, Rocha NP, Simoes ACSE, Teixeira AL, da Silva Camargos ER (2018) Neurotrophic factors in Parkinson’s disease: what have we learned from pre-clinical and clinical studies? Curr Med Chem 25:3682–3702
Rostamian Delavar M, Baghi M, Safaeinejad Z, Kiani-Esfahani A, Ghaedi K, Nasr-Esfahani MH (2018) Differential expression of miR-34a, miR-141, and miR-9 in MPP+-treated differentiated PC12 cells as a model of Parkinson’s disease. Gene 662:54–65
Zhang S, Chen S, Liu A, Wan J, Tang L, Zheng N, Xiong Y (2018) Inhibition of BDNF production by MPP (+) through up-regulation of miR-210-3p contributes to dopaminergic neuron damage in MPTP model. Neurosci Lett 675:133–139
Fu Y, Zhen J, Lu Z (2017) Synergetic neuroprotective effect of docosahexaenoic acid and aspirin in SH-Y5Y by inhibiting miR-21 and activating RXRα and PPARα. DNA Cell Biol 36:482–489
Li D, Yang H, Ma J, Luo S, Chen S, Gu Q (2018) MicroRNA-30e regulates neuroinflammation in MPTP model of Parkinson’s disease by targeting Nlrp3. Hum Cell 31:106–115
Leggio L, Vivarelli S, L’Episcopo F, Tirolo C, Caniglia S, Testa N, Marchetti B, Iraci N (2017) microRNAs in Parkinson’s Disease: From Pathogenesis to Novel Diagnostic and Therapeutic Approaches. Int J Mol Sci 18
Goh SY, Chao YX, Dheen ST, Tan EK, Tay SS (2019) Role of MicroRNAs in Parkinson’s Disease. Int J Mol Sci 20
Li B, Jiang Y, Xu Y, Li Y, Li B (2019) Identification of miRNA-7 as a regulator of brain-derived neurotrophic factor/α-synuclein axis in atrazine-induced Parkinson’s disease by peripheral blood and brain microRNA profiling. Chemosphere 233:542–548
Konovalova J, Gerasymchuk D, Arroyo SN, Kluske S, Mastroianni F, Pereyra AV, Domanskyi A (2021) Human-specific regulation of neurotrophic factors MANF and CDNF by microRNAs. Int J Mol Sci 22:9691
Pradhan J, Noakes PG, Bellingham MC (2019) The role of altered BDNF/TrkB signaling in amyotrophic lateral sclerosis. Front Cell Neurosci 13:368
Beers DR, Zhao W, Liao B, Kano O, Wang J, Huang A, Appel SH, Henkel JS (2011) Neuroinflammation modulates distinct regional and temporal clinical responses in ALS mice. Brain Behav Immun 25:1025–1035
Henriques A, Pitzer C, Schneider A (2010) Neurotrophic growth factors for the treatment of amyotrophic lateral sclerosis: where do we stand? Front Neurosci 4:32
Shruthi S, Sumitha R, Varghese AM, Ashok S, Chandrasekhar Sagar BK, Sathyaprabha TN, Nalini A, Kramer BW et al (2017) Brain-derived neurotrophic factor facilitates functional recovery from ALS-cerebral spinal fluid-induced neurodegenerative changes in the NSC-34 motor neuron cell line. Neurodegener Dis 17:44–58
Parisi C, Napoli G, Amadio S, Spalloni A, Apolloni S, Longone P, Volonté C (2016) MicroRNA-125b regulates microglia activation and motor neuron death in ALS. Cell Death Differ 23:531–541
Rizzuti M, Filosa G, Melzi V, Calandriello L, Dioni L, Bollati V, Bresolin N, Comi GP et al (2018) MicroRNA expression analysis identifies a subset of downregulated miRNAs in ALS motor neuron progenitors. Sci Rep 8:10105
Kovanda A, Leonardis L, Zidar J, Koritnik B, Dolenc-Groselj L, Ristic Kovacic S, Curk T, Rogelj B (2018) Differential expression of microRNAs and other small RNAs in muscle tissue of patients with ALS and healthy age-matched controls. Sci Rep 8:5609
Christoforidou E, Joilin G, Hafezparast M (2020) Potential of activated microglia as a source of dysregulated extracellular microRNAs contributing to neurodegeneration in amyotrophic lateral sclerosis. J Neuroinflammation 17:135
Pradhan J, Noakes PG, Bellingham MC (2019) The role of altered BDNF/TrkB signaling in amyotrophic lateral sclerosis. Front Cell Neurosci 13:368
Gothelf Y, Kaspi H, Abramov N, Aricha R (2017) miRNA profiling of NurOwn®: mesenchymal stem cells secreting neurotrophic factors. Stem Cell Res Ther 8:249
Levy MJF, Boulle F, Steinbusch HW, van den Hove DLA, Kenis G, Lanfumey L (2018) Neurotrophic factors and neuroplasticity pathways in the pathophysiology and treatment of depression. Psychopharmacology 235:2195–2220
Bai M, Zhu X, Zhang Y, Zhang S, Zhang L, Xue L, Yi J, Yao S et al (2012) Abnormal hippocampal BDNF and miR-16 expression is associated with depression-like behaviors induced by stress during early life. PLoS One 7:e46921
Lee M, Cho H, Jung SH, Yim SH, Cho SM, Chun JW, Paik SH, Park YE et al (2018) circulating microRNA expression levels associated with internet gaming disorder. Front Psychiatry 9:81
Mellios N, Huang HS, Baker SP, Galdzicka M, Ginns E, Akbarian S (2009) Molecular determinants of dysregulated GABAergic gene expression in the prefrontal cortex of subjects with schizophrenia. Biol Psychiatry 65:1006–1014
Fang Y, Qiu Q, Zhang S, Sun L, Li G, Xiao S, Li X (2018) Changes in miRNA-132 and miR-124 levels in non-treated and citalopram-treated patients with depression. J Affect Disord 227:745–751
Miyanishi H, Nitta A (2021) A role of BDNF in the depression pathogenesis and a potential target as antidepressant: the modulator of stress sensitivity “Shati/Nat8l-BDNF System” in the dorsal striatum. Pharmaceuticals (Basel) 14
Zhang Y, Zhao Y, Tian C, Wang J, Li W, Zhong C (2018) Differential exosomal microRNA profile in the serum of a patient with depression. Europ J Psychiatry 32:105–112
Yang T, Nie Z, Shu H, Kuang Y, Chen X, Cheng J, Yu S, Liu H (2020) The role of BDNF on neural plasticity in depression. Front Cell Neurosci 14:82
Ihara K, Yoshida H, Jones PB, Hashizume M, Suzuki Y, Ishijima H, Kim HK, Suzuki T et al (2016) Serum BDNF levels before and after the development of mood disorders: a case-control study in a population cohort. Transl Psychiatry 6:e782
Xue Y, Liang H, Yang R, Deng K, Tang M, Zhang M (2021) The role of pro and mature neurotrophins in the depression. Behav Brain Res 404:113162
Ferrúa CP, Giorgi R, da Rosa LC, da Amaral CC, Ghisleni GC, Pinheiro RT, Nedel F (2019) MicroRNAs expressed in depression and their associated pathways: a systematic review and a bioinformatics analysis. J Chem Neuroanat 100:101650
Lian N, Niu Q, Lei Y, Li X, Li Y, Song X (2018) MiR-221 is involved in depression by regulating Wnt2/CREB/BDNF axis in hippocampal neurons. Cell Cycle 17:2745–2755
Wang SS, Mu RH, Li CF, Dong SQ, Geng D, Liu Q, Yi LT (2017) microRNA-124 targets glucocorticoid receptor and is involved in depression-like behaviors. Prog Neuropsychopharmacol Biol Psychiatry 79:417–425
Yi LT, Mu RH, Dong SQ, Wang SS, Li CF, Geng D, Liu Q (2018) miR-124 antagonizes the antidepressant-like effects of standardized gypenosides in mice. J Psychopharmacol 32:458–468
Bahi A, Chandrasekar V, Dreyer JL (2014) Selective lentiviral-mediated suppression of microRNA124a in the hippocampus evokes antidepressants-like effects in rats. Psychoneuroendocrinology 46:78–87
Yang X, Yang Q, Wang X, Luo C, Wan Y, Li J, Liu K, Zhou M et al (2014) MicroRNA expression profile and functional analysis reveal that miR-206 is a critical novel gene for the expression of BDNF induced by ketamine. Neuromolecular Med 16:594–605
Li YJ, Xu M, Gao ZH, Wang YQ, Yue Z, Zhang YX, Li XX, Zhang C et al (2013) Alterations of serum levels of BDNF-related miRNAs in patients with depression. PLoS One 8:e63648
Xin C, Xia J, Liu Y, Zhang Y (2020) MicroRNA-202-3p Targets brain-derived neurotrophic factor and is involved in depression-like behaviors. Neuropsychiatr Dis Treat 16:1073–1083
Su M, Hong J, Zhao Y, Liu S, Xue X (2015) MeCP2 controls hippocampal brain-derived neurotrophic factor expression via homeostatic interactions with microRNA 132 in rats with depression. Mol Med Rep 12:5399–5406
Li Y, Fan C, Wang L, Lan T, Gao R, Wang W, Yu SY (2021) MicroRNA-26a-3p rescues depression-like behaviors in male rats via preventing hippocampal neuronal anomalies. J Clin Invest 131
Li Y, Li S, Yan J, Wang D, Yin R, Zhao L, Zhu Y, Zhu X (2016) miR-182 (microRNA-182) suppression in the hippocampus evokes antidepressant-like effects in rats. Prog Neuropsychopharmacol Biol Psychiatry 65:96–103
Suliman S, Hemmings SM, Seedat S (2013) Brain-Derived Neurotrophic Factor (BDNF) protein levels in anxiety disorders: systematic review and meta-regression analysis. Front Integr Neurosci 7:55
Muiños-Gimeno M, Guidi M, Kagerbauer B, Martín-Santos R, Navinés R, Alonso P, Menchón JM, Gratacòs M et al (2009) Allele variants in functional MicroRNA target sites of the neurotrophin-3 receptor gene (NTRK3) as susceptibility factors for anxiety disorders. Hum Mutat 30:1062–1071
Murphy CP, Singewald N (2019) Role of MicroRNAs in anxiety and anxiety-related disorders. Curr Top Behav Neurosci 42:185–219
Gören JL (2016) Brain-derived neurotrophic factor and schizophrenia. Ment Health Clin 6:285–288
Cao T, Zhen XC (2018) Dysregulation of miRNA and its potential therapeutic application in schizophrenia. CNS Neurosci Ther 24:586–597
Thomas KT, Anderson BR, Shah N, Zimmer SE, Hawkins D, Valdez AN, Gu Q, Bassell GJ (2017) Inhibition of the schizophrenia-associated microRNA miR-137 disrupts Nrg1α neurodevelopmental signal transduction. Cell Rep 20:1–12
Mellios N, Sur M (2012) The emerging role of microRNAs in schizophrenia and autism spectrum disorders. Front Psychiatry 3:39
Pan S, Feng W, Li Y, Huang J, Chen S, Cui Y, Tian B, Tan S et al (2021) The microRNA-195 - BDNF pathway and cognitive deficits in schizophrenia patients with minimal antipsychotic medication exposure. Transl Psychiatry 11:117
Perkins DO, Jeffries CD, Jarskog LF, Thomson JM, Woods K, Newman MA, Parker JS, Jin J et al (2007) microRNA expression in the prefrontal cortex of individuals with schizophrenia and schizoaffective disorder. Genome Biol 8:R27
Wu H, Tao J, Chen PJ, Shahab A, Ge W, Hart RP, Ruan X, Ruan Y et al (2010) Genome-wide analysis reveals methyl-CpG-binding protein 2-dependent regulation of microRNAs in a mouse model of Rett syndrome. Proc Natl Acad Sci USA 107:18161–18166
Guo AY, Sun J, Jia P, Zhao Z (2010) A Novel microRNA and transcription factor mediated regulatory network in schizophrenia. BMC Syst Biol 4:10
Eldredge LC, Gao XM, Quach DH, Li L, Han X, Lomasney J, Tourtellotte WG (2008) Abnormal sympathetic nervous system development and physiological dysautonomia in Egr3-deficient mice. Development 135:2949–2957
Sequeira-Cordero A, Brenes JC (2021) Time-dependent changes in striatal monoamine levels and gene expression following single and repeated amphetamine administration in rats. Eur J Pharmacol 904:174148
Ehinger Y, Phamluong K, Darevesky D, Welman M, Moffat JJ, Sakhai SA, Whiteley EL, Berger AL et al (2021) Differential correlation of serum BDNF and microRNA content in rats with rapid or late onset of heavy alcohol use. Addict Biol 26:e12890
Kyzar EJ, Bohnsack JP, Zhang H, Pandey SC (2019) MicroRNA-137 drives epigenetic reprogramming in the adult amygdala and behavioral changes after adolescent alcohol exposure. eNeuro 6:ENEURO0401
Solomon MG, Griffin WC, Lopez MF, Becker HC (2019) Brain regional and temporal changes in BDNF mRNA and microRNA-206 expression in mice exposed to repeated cycles of chronic intermittent ethanol and forced swim stress. Neuroscience 406:617–625
Tapocik JD, Barbier E, Flanigan M, Solomon M, Pincus A, Pilling A, Sun H, Schank JR et al (2014) microRNA-206 in rat medial prefrontal cortex regulates BDNF expression and alcohol drinking. J Neurosci 34:4581–4588
Xin F, Ye XM, Liu HB, Liu LP, Yan LJ, Hu J (2018) Differential expression and analysis of target regulation of microRNAs in alcohol-dependent rats. J Biol Regul Homeost Agents 32:825–841
Darcq E, Warnault V, Phamluong K, Besserer GM, Liu F, Ron D (2015) MicroRNA-30a-5p in the prefrontal cortex controls the transition from moderate to excessive alcohol consumption. Mol Psychiatry 20:1219–1231
Yang X, Yang Q, Wang X, Luo C, Wan Y, Li J, Liu K, Zhou M et al (2014) MicroRNA expression profile and functional analysis reveal that miR-206 is a critical novel gene for the expression of BDNF induced by ketamine. Neuromolecular Med 16:594–605
Shen Q, Xie B, Galaj E, Yu H, Li X, Lu Y, Zhang M, Wen D et al (2022) CircTmeff-1 in the nucleus accumbens regulates the reconsolidation of cocaine-associated memory. Brain Res Bull 185:64–73
Bastle RM, Oliver RJ, Gardiner AS, Pentkowski NS, Bolognani F, Allan AM, Chaudhury T, St Peter M et al (2018) In silico identification and in vivo validation of miR-495 as a novel regulator of motivation for cocaine that targets multiple addiction-related networks in the nucleus accumbens. Mol Psychiatry 23:434–443
Im HI, Hollander JA, Bali P, Kenny PJ (2010) MeCP2 controls BDNF expression and cocaine intake through homeostatic interactions with microRNA-212. Nat Neurosci 13:1120–1127
Dreyer JL (2010) New insights into the roles of microRNAs in drug addiction and neuroplasticity. Genome Med 2:92
Bali P, Kenny PJ (2013) MicroRNAs and drug addiction. Front Genet 4:43
Chandrasekar V, Dreyer JL (2009) microRNAs miR-124, let-7d and miR-181a regulate cocaine-induced plasticity. Mol Cell Neurosci 42:350–362
Maurel OM, Torrisi SA, Barbagallo C, Purrello M, Salomone S, Drago F, Ragusa M, Leggio GM (2021) Dysregulation of miR-15a-5p, miR-497a-5p and miR-511-5p is associated with modulation of BDNF and FKBP5 in brain areas of PTSD-related susceptible and resilient mice. Int J Mol Sci 22:5157
Tong L, Li MD, Nie PY, Chen Y, Chen YL, Ji LL (2021) miR-132 downregulation alleviates behavioral impairment of rats exposed to single prolonged stress, reduces the level of apoptosis in PFC, and upregulates the expression of MeCP2 and BDNF. Neurobiol Stress 14:100311
Ji LL, Ye Y, Nie PY, Peng JB, Fu CH, Wang ZY, Tong L (2019) Dysregulation of miR-142 results in anxiety-like behaviors following single prolonged stress. Behav Brain Res 365:157–163
Wang Z, Zhang C, Huang J, Yuan C, Hong W, Chen J, Yu S, Xu L et al (2014) MiRNA-206 and BDNF genes interacted in bipolar I disorder. J Affect Disord 162:116–119
Lee SY, Wang TY, Lu RB, Wang LJ, Chang CH, Chiang YC, Tsai KW (2021) Peripheral BDNF correlated with miRNA in BD-II patients. J Psychiatr Res 136:184–189
Alural B, Ozerdem A, Allmer J, Genc K, Genc S (2015) Lithium protects against paraquat neurotoxicity by NRF2 activation and miR-34a inhibition in SH-SY5Y cells. Front Cell Neurosci 9:209
Acknowledgements
Not applicable.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
S.A., Data Collection, Writing— Original draft preparation; A.Z., Data Collection, Writing, Original draft preparation; F.N., Conceptualization, Writing—Review & Editing; A.G., Conceptualization, Writing—Review & Editing.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
All authors have read and agreed to the published version of the manuscript.
Competing Interests
The authors declare no competing interests.
Research involving Human Participants and/or Animals
Not applicable.
Informed Consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdolahi, S., Zare-Chahoki, A., Noorbakhsh, F. et al. A Review of Molecular Interplay between Neurotrophins and miRNAs in Neuropsychological Disorders. Mol Neurobiol 59, 6260–6280 (2022). https://doi.org/10.1007/s12035-022-02966-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-02966-5