Abstract
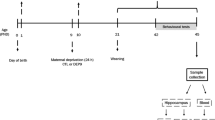
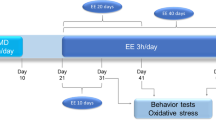
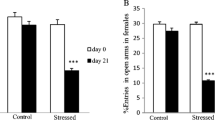
This study aimed at evaluating the treatment effects with ketamine, electroconvulsive stimulation (ECS), escitalopram, alone or in combination in adult rats of both sexes, subjected to the animal model of maternal deprivation (MD). All groups were subjected to the forced swimming test (FST), splash and open field tests. The prefrontal cortex (PFC), hippocampus and serum were collected to analyze oxidative stress and inflammatory parameters. MD induced depressive-like behavior in the FST test in males and reduced grooming time in male and female rats. The treatments alone or combined reversed depressive and anhedonic behavior in females. In males, all treatments increased grooming time, except for ECS + escitalopram + ketamine. MD increased lipid peroxidation and protein carbonylation, nitrite/nitrate concentration and myeloperoxidase activity in the PFC and hippocampus of males and females. However, the treatment’s response was sex dependent. Catalase activity decreased in the PFC of males and the PFC and hippocampus of females, and most treatments were not able to reverse it. MD increased the inflammation biomarkers levels in the PFC and hippocampus of males and females, and most treatments were able to reverse this increase. In all groups, a reduction in the interleukin-10 levels in the PFC and hippocampus of female and male rats was observed. Our study shows different responses between the sexes in the patterns evaluated and reinforces the use of the gender variable as a biological factor in MDD related to early stress and in the response of the therapeutic strategies used.










Similar content being viewed by others
Data availability
The data will be made available on reasonable request.
References
Greenberg PE, Fournier AA, Sisitsky T, Pike CT, Kessler RC (2015) The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry 76:155–162. https://doi.org/10.4088/JCP.14m09298
Beurel E, Toups M, Nemeroff CB (2020) The Bidirectional Relationship of Depression and Inflammation: Double Trouble. Neuron 107:234–256. https://doi.org/10.1016/j.neuron.2020.06.002
Salk RH, Hyde JS, Abramson LY (2017) Gender differences in depression in representative national samples: Meta-analyses of diagnoses and symptoms. Psychol Bull 143:783–822. https://doi.org/10.1037/bul0000102
Ferrari AJ, Somerville AJ, Baxter AJ, Norman R, Patten SB, Vos R, Whiteford HA (2013) Global variation in the prevalence and incidence of major depressive disorder: a systematic review of the epidemiological literature. Psychol Med 43:471–481. https://doi.org/10.1017/S0033291712001511
Lindqvist D, Dhabhar FS, James SJ, Hough CM, Jain FA, Bersani FS, Reus VI, Verhoeven E et al (2017) Oxidative stress, inflammation and treatment response in major depression. Psychoneuroendocrinol 76:197–205. https://doi.org/10.1016/j.psyneuen.2016.11.031
Hindmarch I (2002) Beyond the monoamine hypothesis: mechanisms, molecules and methods. Eur Psychiatry 17:294–299. https://doi.org/10.1016/s0924-9338(02)00653-3
Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB (2008) The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinol 33:693–710. https://doi.org/10.1016/j.psyneuen.2008.03.008
Nemeroff CB (1999) The preeminent role of early untoward experience on vulnerability to major psychiatric disorders: the nature-nurture controversy revisited and soon to be resolved. Mol Psychiatry 4:106. https://doi.org/10.1038/sj.mp.4000512
Nemeroff CB, Heim CM, Thase ME, Klein DN, Rush AJ, Schatzberg AF, Keller MB (2003) Differential responses to psychotherapy versus pharmacotherapy in patients with chronic forms of major depression and childhood trauma. Proc Natl Acad Sci 100:14293–14296. https://doi.org/10.1073/pnas.2336126100
Gold PW (2014) The organization of the stress system and its dysregulation in depressive illness. Mol Psychiatry 20:32. https://doi.org/10.1038/mp.2014.163
Averill LA, Abdallah CG, Fenton LR, Fasula MK, Jiang L, Rothman DL, Mason GF, Sanacora G (2020) Early life stress and glutamate neurotransmission in major depressive disorder. Eur Neuropsychopharmacol 35:71–80. https://doi.org/10.1016/j.euroneuro.2020.03.015
Czéh B, Fuchs E, Wiborg O, Simon M (2016) Animal models of major depression and their clinical implications. Prog Neuropsychopharmacol Biol Psychiatry 64:293–310. https://doi.org/10.1016/j.pnpbp.2015.04.004
Réus GZ, Abelaira HM, dos Santos MA, Carlessi AS, Tomaz DB, Neotti MV, Liranço JL, Gubert C et al (2013) Ketamine and imipramine in the nucleus accumbens regulate histone deacetylation induced by maternal deprivation and are critical for associated behaviors. Behav Brain Res 256:451–456. https://doi.org/10.1016/j.bbr.2013.08.041
Réus GZ, Carlessi AS, Titus SE, Abelaira HM, Ignácio ZM, da Luz JR, Matias BI, Bruchchen L et al (2015) A single dose of S-ketamine induces long-term antidepressant effects and decreases oxidative stress in adulthood rats following maternal deprivation. Dev Neurobiol 75:1268–1281. https://doi.org/10.1002/dneu.22283
Réus GZ, Silva RH, de Moura AB, Presa JF, Abelaira HM, Abatti M, Vieira A, Pescador B et al (2019) Early Maternal Deprivation Induces Microglial Activation, Alters Glial Fibrillary Acidic Protein Immunoreactivity and Indoleamine 2,3-Dioxygenase during the Development of Offspring Rats. Mol Neurobiol 56:1096–1108. https://doi.org/10.1007/s12035-018-1161-2
Kurutas EB (2016) The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state. Nutr J 15:71. https://doi.org/10.1186/s12937-016-0186-5
Salim S (2017) Oxidative stress and the central nervous system. J Pharmacol Exp Ther 360:201–205. https://doi.org/10.1124/jpet.116.237503
Joshi YB, Pratico D (2014) Lipid peroxidation in psychiatric illness: overview Q16 of clinical evidence. Oxid Med Cell Longev XX, 828702https://doi.org/10.1155/2014/828702
Che Y, Zhou Z, Shu Y, Zhai C, Zhu Y, Gong S, Cui Y, Wang JF (2015) Chronic unpredictable stress impairs endogenous antioxidant defense in rat brain. Neurosci Lett 584:208–213. https://doi.org/10.1016/j.neulet.2014.10.031
Visentin APV, Colombo R, Scotton E, Fracasso DS, da Rosa AR, Branco CS, Salvador M (2020) Targeting Inflammatory-Mitochondrial Response in Major Depression: Current Evidence and Further Challenges. Oxid Med Cell Longev 2020:2972968. https://doi.org/10.1155/2020/2972968
Schiepers OJ, Wichers MC, Maes M (2005) Cytokines and major depression. Prog Neuropsychopharmacol Biol Psychiatry 29:201–217. https://doi.org/10.1016/j.pnpbp.2004.11.003
Köhler CA, Freitas TH, Maes M, de Andrade NQ, Liu CS, Fernandes BS, Stubbs B, Solmi M et al (2017) Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatr Scand 135:373–387. https://doi.org/10.1111/acps.12698 (Epub 2017 Jan 25)
Thomas SJ, Shin M, McInnis MG, Bostwick JR (2015) Combination therapy with monoamine oxidase inhibitors and other antidepressants or stimulants: strategies for the management of treatment-resistant depression. Pharmacotherapy 35:433–449. https://doi.org/10.1002/phar.1576
Réus GZ, Fernandes GC, de Moura AB, Silva RH, Darabas AC, de Souza TG, Abelaira HM, Carneiro C et al (2017) Early life experience contributes to the developmental programming of depressive-like behaviour, neuroinflammation and oxidative stress. J Psychiatr Res 95:196–207. https://doi.org/10.1016/j.jpsychires.2017.08.020
Olesen MV, Wörtwein G, Folke J, Pakkenberg B (2017) Electroconvulsive stimulation results in long-term survival of newly generated hippocampal neurons in rats. Hippocampus 27:52–60. https://doi.org/10.1002/hipo.22670
Dionisie V, Ciobanu AM, Toma VA, Manea MC, Baldea I, Olteanu D, Sevastre-Berghian A, Clichici S et al (2021) Escitalopram Targets Oxidative Stress, Caspase-3, BDNF and MeCP2 in the Hippocampus and Frontal Cortex of a Rat Model of Depression Induced by Chronic Unpredictable Mild Stress. Int J Mol Sci 22(14):7483. https://doi.org/10.3390/ijms22147483
Réus GZ, Stringari RB, Ribeiro KF, Cipriano AL, Panizzutti BS, Stertz L, Lersch C, Kapczinski F et al (2011) Maternal deprivation induces depressive-like behaviour and alters neurotrophin levels in the rat brain. Neurochem Res 36:460–466. https://doi.org/10.1007/s11064-010-0364-3
Isingrini E, Camus V, Le Guisquet AM, Pingaud M, Devers S, Belzung C (2010) Association between repeated unpredictable chronic mild stress (UCMS) procedures with a high fat diet: a model of fluoxetine resistance in mice. PLoS One 5:e10404. https://doi.org/10.1371/journal.pone.0010404
Porsolt RD, Bertin A, Jalfre M (1977) Behavioural despair in mice: a primary screening test for antidepressants. Archive International Pharmacodyn Therapy 229:27–336
Paxinos G, Watson C (1986) The Rat Brain: Stereotaxic Coordinates, 2nd edn. Academic Press, Australia
Esterbauer H (1990) Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol 21:186–207. https://doi.org/10.1016/0076-6879(90)86134-h
Levine RL, Garland D, Oliver CN (1990) Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol 186:464–478. https://doi.org/10.1016/0076-6879(90)86141-h
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982) Analysis of nitrate, nitrite, and [15 N] nitrate in biological fluids. Anal Biochem 126:131–138. https://doi.org/10.1016/0003-2697(82)90118-x
Bannister JV, Calabrese L (1987) Assays for superoxide dismutase. Met Biochem Anal 32:279–312. https://doi.org/10.1002/9780470110539.ch5
De Young LM, Kheifets JB, Ballaron SJ, Young JM (1989) Edema and cell infiltration in the phorbol ester-treated mouse ear are temporally separate and can be differentially modulated by pharmacologic agents. Agents Actions 126:335–341. https://doi.org/10.1007/BF01967298
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Goodwill HL, Manzano-Nieves G, Gallo M, Lee HI, Oyerinde E, Serre T, Bath KG (2019) Early life stress leads to sex differences in development of depressive-like outcomes in a mouse model. Neuropsychopharmacol 44:711–720. https://doi.org/10.1038/s41386-018-0195-5
Borba LA, Broseghini LDR, Manosso LM, de Moura AB, Botelho MEM, Arent CO, Behenck JP, Hilsendeger A et al (2021) Environmental enrichment improves lifelong persistent behavioral and epigenetic changes induced by early-life stress. J Psychiatr Res 138:107–116. https://doi.org/10.1016/j.jpsychires.2021.04.008
Abelaira HM, Veron DC, de Moura AB, Carlessi AS, Borba LA, Botelho MEMB, Andrade NM, Martinello NS et al (2021) Sex differences on the behavior and oxidative stress after ketamine treatment in adult rats subjected to early life stress. Brain Res Bull S0361–9230:00124–00126. https://doi.org/10.1016/j.brainresbull.2021.04.021
Thelen C, Sens J, Mauch J, Pandit R, Pitychoutis PM (2016) Repeated ketamine treatment induces sex-specific behavioral and neurochemical effects in mice. Behav Brain Res 312:305–312. https://doi.org/10.1016/j.bbr.2016.06.041
McDougall SA, Park GI, Ramirez GI, Gomez V, Adame BC, Crawford CA (2019) Sex-dependent changes in ketamine-induced locomotor activity and ketamine pharmacokinetics in preweanling, adolescent, and adult rats. Eur Neuropsychopharmacol 29:740–755. https://doi.org/10.1016/j.euroneuro.2019.03.013
McDougall SA, Moran AE, Baum TJ, Apodaca MG, Real V (2017) Effects of ketamine on the unconditioned and conditioned locomotor activity of preadolescent and adolescent rats: impact of age, sex, and drug dose. Psychopharmacol 234:2683–2696. https://doi.org/10.1007/s00213-017-4660-3
Wilson C, Cone K, Kercher M, Hibbitts J, Fischer J, Van Lake A, Sumner J (2005) Naloxone increases ketamine-induced hyperactivity in the open field in female rats. Pharmacol Biochem Behav 81:530–534. https://doi.org/10.1016/j.pbb.2005.03.018
Wilson C, Kercher M, Quinn B, Murphy A, Fiegel C, McLaurin A (2007) Effects of age and sex on ketamine-induced hyperactivity in rats. Physiol Behav 91:202–207. https://doi.org/10.1016/j.physbeh.2007.02.010
Carrier N, Kabbaj M (2013) Sex differences in the antidepressant-like effects of ketamine. Neuropharmacol 70:27–34. https://doi.org/10.1016/j.neuropharm.2012.12.009
Franceschelli A, Sens J, Herchick S, Thelen C, Pitychoutis PM (2015) Sex differences in the rapid and the sustained antidepressant-like effects of ketamine in stress-naive and "depressed" mice exposed to chronic mild stress. Neurosci 49-60https://doi.org/10.1016/j.neuroscience.2015.01.008
Frye C, Walf A (2002) Changes in progesterone metabolites in the hippocampus can modulate open field and forced swim test behavior of proestrous rats. Horm Behav 41:306–315. https://doi.org/10.1006/hbeh.2002.1763
Greenhalgh J, Knight C, Hind D, Beverley C, Walters S (2005) Clinical and cost-effectiveness of electroconvulsive therapy for depressive illness, schizophrenia, catatonia and mania: systematic reviews and economic modelling studies. Health Technol Assess 9:1e156.iiieiv. https://doi.org/10.3310/hta9090
Güney P, Ekman CJ, Hammar Å, Heintz E, Landén M, Lundberg J, Nordanskog P, Nordenskjöld A (2020) Electroconvulsive Therapy in Depression: Improvement in Quality of Life Depending on Age and Sex. J ECT 36:242–246. https://doi.org/10.1097/YCT.0000000000000671
Socała K, Nieoczym D, Wyska E, Wlaź P (2017) Effect of sildenafil on the activity of some antidepressant drugs and electroconvulsive shock treatment in the forced swim test in mice. Naunyn Schmiedebergs Arch Pharmacol 390:339–349. https://doi.org/10.1007/s00210-016-1334-3
Khan A, Brodhead AE, Schwartz KA, Kolts RL, Brown WA (2005) Sex differences in antidepressant response in recent antidepressant clinical trials. J Clin Psychopharmacol 25:318–324. https://doi.org/10.1097/01.jcp.0000168879.03169.ce
Yalcin I, Aksu F, Belzung C (2005) Effects of desipramine and tramadol in a chronic mild stress model in mice are altered by yohimbine but not by pindolol. Eur J Pharmacol 514:165–174. https://doi.org/10.1016/j.ejphar.2005.03.029
Zhang T, He K, Bai T, Lv H, Xie X, Nie J, Xie W, Zhu C et al (2020) Altered neural activity in the reward-related circuit and executive control network associated with amelioration of anhedonia in major depressive disorder by electroconvulsive therapy. Prog Neuropsychopharmacol Biol Psychiatry 109:110193. https://doi.org/10.1016/j.pnpbp.2020.110193
Henningsen K, Woldbye DP, Wiborg O (2013) Electroconvulsive stimulation reverses anhedonia and cognitive impairments in rats exposed to chronic mild stress. Eur Neuropsychopharmacol 23:1789–1794. https://doi.org/10.1016/j.euroneuro.2013.03.011
Burstein O, Franko M, Gale E, Handelsman A, Barak S, Motsan S, Shamir A, Toledano R et al (2017) Escitalopram and NHT normalized stress-induced anhedonia and molecular neuroadaptations in a mouse model of depression. PLoS One 12:e0188043. https://doi.org/10.1371/journal.pone.0188043
Jha MK, Minhajuddin A, Gadad BS, Chin Fatt C, Trivedi MH (2019) Higher S100B Levels Predict Persistently Elevated Anhedonia with Escitalopram Monotherapy Versus Antidepressant Combinations: Findings from CO-MED Trial. Pharmaceuticals (Basel) 12:184. https://doi.org/10.3390/ph12040184
Gałuszko-Węgielnik M, Wiglusz MS, Słupski J, Szałach Ł, Włodarczk A, Górska N, Szarmach J, Jakuszkowiak-Wojten K et al (2019) Efficacy of Ketamine in bipolar depression: focus on anhedonia. Psychiatr Danub 31:554–560
Ballard ED, Wills K, Lally N, Richards EM, Luckenbaugh DA, Walls T, Ameli R, Niciu MJ et al (2017) Anhedonia as a clinical correlate of suicidal thoughts in clinical ketamine trials. J Affect Disord 218:195–200. https://doi.org/10.1016/j.jad.2017.04.057
Lally N, Nugent AC, Luckenbaugh DA, Ameli R, Roiser JP, Zarate CA (2014) Anti-anhedonic effect of ketamine and its neural correlates in treatment-resistant bipolar depression. Transl Psychiatry 4:e469. https://doi.org/10.1038/tp.2014.105
Tenkorang MA, Snyder B, Cunningham RL (2018) Sex-related differences in oxidative stress and neurodegeneration. Steroids 133:21–27. https://doi.org/10.1016/j.steroids.2017.12.010
Wiener C, Rassier GT, Kaster MP, Jansen K, Pinheiro RT, Klamt F, Magalhães PV, Kapczinski F et al (2014) Gender-based differences in oxidative stress parameters do not underlie the differences in mood disorders susceptibility between sexes. Eur Psychiatry 29:58–63. https://doi.org/10.1016/j.eurpsy.2013.05.006
Wright KN, Kabbaj M (2018) Sex differences in sub-anesthetic ketamine’s antidepressant effects and abuse liability. Curr Opin Behav Sci 23:36–41. https://doi.org/10.1016/j.cobeha.2018.02.001
Chen WY, Huang MC, Lin SK (2014) Gender differences in subjective discontinuation symptoms associated with ketamine use. Subst Abuse Treat Prev Policy 9:39. https://doi.org/10.1186/1747-597X-9-39
Neves BH, Menezes J, Souza MA, Mello-Carpes PB (2015) Physical exercise prevents short and long-term deficits on aversive and recognition memory and attenuates brain oxidative damage induced by maternal deprivation. Physiol Behav 152:99–105. https://doi.org/10.1016/j.physbeh.2015.09.019
Réus GZ, Nacif MP, Abelaira HM, Tomaz DB, dos Santos MA, Carlessi AS, da Luz JR, Gonçalves RC et al (2015) Ketamine ameliorates depressive-like behaviors and immune alterations in adult rats following maternal deprivation. Neurosci Lett 584:83–87. https://doi.org/10.1016/j.neulet.2014.10.022
Shalaby A, Kamal S (2009) Effect of Escitalopram on GABA level and anti-oxidant markers in prefrontal cortex and nucleus accumbens of chronic mild stress-exposed albino rats. Int J Physiol Pathophysiol Pharmacol 1:154–161
Jornada LK, Feier G, Barichello T, Vitali ÂM, Reinke A, Gavioli EC et al (2007) Effects of maintenance electroshock on the oxidative damage parameters in the rat brain. Neurochem Res 32:389–394. https://doi.org/10.1007/s11064-006-9214-8
Barichello T, Bonatto F, Agostinho FR, Reinke A, Moreira JCF, Dal-Pizzol F et al (2004) Structure-related oxidative damage in rat brain after acute and chronic electroshock. Neurochem Res 29:1749–1753. https://doi.org/10.1023/b:nere.0000035811.06277.b3
De Oliveira L, Cecília CM, Bortolin T, Canever L, Petronilho F, Gonçalves Mina F et al (2009) Different sub-anesthetic doses of ketamine increase oxidative stress in the brain of rats. Prog Neuro-Psychopharmacology Biol Psychiatry 33:1003–1008. https://doi.org/10.1016/j.pnpbp.2009.05.010
Réus GZ, Abaleira HM, Titus SE, Arent CO, Michels M, Da Luz JR et al (2016) Effects of ketamine administration on the phosphorylation levels of CREB and TrKB and on oxidative damage after infusion of MEK inhibitor. Pharmacol Reports 68:177–184. https://doi.org/10.1016/j.pharep.2015.08.010
Maciel AL, Abelaira HM, de Moura AB, de Souza TG, Rosa T, Matos D, Tuon T, Garbossa L et al (2018) Acute treatment with ketamine and chronic treatment with minocycline exert antidepressant-like effects and antioxidant properties in rats subjected different stressful events. Brain Res Bull 137:204–216. https://doi.org/10.1016/j.brainresbull.2017.12.005
Slamon ND, Pentreath VW (2000) Antioxidant defense against antidepressants in C6 and 1321N1 cells. Chem Biol Interact 127:181–199. https://doi.org/10.1016/s0009-2797(00)00172-1
Liu Y, Lan N, Ren J, Wu Y, Wang ST, Huang XF, Yu Y (2015) Orientin improves depression-like behavior and BDNF in chronic stressed mice. Mol Nutr Food Res 59:1130–1142. https://doi.org/10.1002/mnfr.201400753
Ortmann CF, Réus GZ, Ignácio ZM, Abelaira HM, Titus SE, de Carvalho P, Arent CO, Dos Santos MA et al (2016) Enriched Flavonoid Fraction from Cecropia pachystachya Trécul Leaves Exerts Antidepressant-like Behavior and Protects Brain Against Oxidative Stress in Rats Subjected to Chronic Mild Stress. Neurotox Res 29:469–483. https://doi.org/10.1007/s12640-016-9596-6
Cimen B, Gumus CB, Cetin I, Ozsoy S, Aydin M, Cimen L (2015) The Effects of Escitalopram Treatment on Oxidative/ Antioxidative Parameters in Patients with Depression. Bull of Clin Psychopharmacol 3:272–279
Lv F, Shen YW, Peng LH, Li P, Luo J, Wei K, Li W, Chen J et al (2013) Effects of propofol on expression of hippocampal neuronal nitric oxide synthase and carboxy-terminal PDZ ligand of neuronal nitric oxide synthase in stressed rats undergoing electroconvulsive shock. J ECT 29:297–302. https://doi.org/10.1097/YCT.0b013e318290fa17
Soubhye J, Aldib I, Prévost M, Elfving B, Gelbcke M, Podrecca M, Conotte R, Colet JM et al (2014) Pharm Pharmacol 66:1122–1132. https://doi.org/10.1111/jphp.12236
Yamamori H, Ishima T, Yasuda Y, Fujimoto M, Kudo N, Ohi K, Hashimoto K, Takeda M et al (2016) Assessment of a multi-assay biological diagnostic test for mood disorders in a Japanese population. Neurosci Lett 612:167–171. https://doi.org/10.1016/j.neulet.2015.12.019
Vaccarino V, Brennan ML, Miller AH, Bremner JD, Ritchie JC, Lindau F, Veledar E, Su S et al (2008) Association of major depressive disorder with serum myeloperoxidase and other markers of inflammation: a twin study. Biol Psychiatry 64:476–483. https://doi.org/10.1016/j.biopsych.2008.04.023
Kartalci S, Karabulut AB, Erbay LG, Acar C (2016) Effects of Electroconvulsive Therapy on Some Inflammatory Factors in Patients With Treatment-Resistant Schizophrenia. J ECT 32:174–179. https://doi.org/10.1097/YCT.0000000000000303
Abdo SA, Wadie W, Abdelsalam RM, Khattab MM (2019) Potential Anti-Inflammatory Effect of Escitalopram in Iodoacetamide-Induced Colitis in Depressed Ovariectomized Rats: Role of α7-nAChR. Inflammation 42:2056–2064. https://doi.org/10.1007/s10753-019-01068-0
Maes M, Yirmyia R, Noraberg J, Brene S, Hibbeln J, Perini G, Kubera M, Bob P et al (2009) The inflammatory & neurodegenerative (I&ND) hypothesis of depression: leads for future research and new drug developments in depression. Metab Brain Dis 24:27–53. https://doi.org/10.1007/s11011-008-9118-1
Miller AH, Maletic V, Raison CL (2009) Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry 65:732–741. https://doi.org/10.1016/j.biopsych.2008.11.029
Ménard C, Pfau ML, Hodes GE, Russo SJ (2017) Immune and neuroendocrine mechanisms of stress vulnerability and resilience. Neuropsychopharmacology 42:62–80. https://doi.org/10.1038/npp.2016.90
Miller AH, Raison CL (2016) The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol 16:22–34. https://doi.org/10.1038/nri.2015.5
Bekhbat M, Neigh GN (2018) Sex differences in the neuro-immune consequences of stress: Focus on depression and anxiety. Brain Behav Immun 67:1–12. https://doi.org/10.1016/j.bbi.2017.02.006
Klein SL (2016) Flanagan KL. Sex differences in immune responses. Nat rev Immunol 16:626–638. https://doi.org/10.1038/nri.2016.90
Ye Y, Yao S, Wang R, Fang Z, Zhong K, Nie L, Zhang Q (2019) PI3K/Akt/NF-kappaB signaling pathway regulates behaviors in adolescent female rats following with neonatal maternal deprivation and chronic mild stress. Behav Brain Res 362:199–207. https://doi.org/10.1016/j.bbr.2019.01.008
Wang Q, Dong X, Wang Y, Liu M, Sun A, Li N, Lin Y, Geng Z et al (2017) Adolescent escitalopram prevents the effects of maternal separation on depression- and anxiety-like behaviours and regulates the levels of inflammatory cytokines in adult male mice. Int J Dev Neurosci 62:37–45. https://doi.org/10.1016/j.ijdevneu.2017.07.007
Yang C, Hong T, Shen J, Ding J, Dai XW, Zhou ZQ, Yang JJ (2013) Ketamine exerts antidepressant effects and reduces IL-1beta and IL-6 levels in rat prefrontal cortex and hippocampus. Exp Ther Med 5:1093–1096. https://doi.org/10.3892/etm.2013.930
Gonçalves CL, Abelaira HM, Rosa T, de Moura AB, Veron DC, Borba LA, Botelho MEM, Goldim MP et al (2021) Ketamine treatment protects against oxidative damage and the immunological response induced by electroconvulsive therapy. Pharmacol Rep 73:525–535. https://doi.org/10.1007/s43440-020-00200-4
Baumeister D, Akhtar R, Ciufolini S, Pariante CM, Mondelli V (2016) Childhood trauma and adulthood inflammation: a meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-α. Mol Psychiatry 21:642–649. https://doi.org/10.1038/mp.2015.67
Järventausta K, Sorri A, Kampman O, Björkqvist M, Tuohimaa K, Hämäläinen M et al (2017) Changes in interleukin-6 levels during electroconvulsive therapy may reflect the therapeutic response in major depression. Acta Psychiatr Scand 135:87–92. https://doi.org/10.1111/acps.12665
Kranaster L, Hoyer C, Aksay SS, Bumb JM, Müller N, Zill P, et al (2017) Antidepressant efficacy of electroconvulsive therapy is associated with a reduction of the innate cellular immune activity in the cerebrospinal fluid in patients with depression World J Biol. Psychiatry 1-11https://doi.org/10.1080/15622975.2017.1355473
Zincir S, Öztürk P, Bilgen AE, İzci F, Yükselir C (2016) Levels of serum immunomodulators and alterations with electroconvulsive therapy in treatment-resistant major depression. Neuropsychiatr Dis Treat 12:1389–1396. https://doi.org/10.2147/NDT.S106652
Rush G, O’Donovan A, Nagle L, Conway C, McCrohan A, O’Farrelly C, Lucey JV, Malone KMJ (2016) Alteration of immune markers in a group of melancholic depressed patients and their response to electroconvulsive therapy. Affect Disord 205:60–68. https://doi.org/10.1016/j.jad.2016.06.035
Fluitman SB, Heijnen CJ, Denys DA, Nolen WA, Balk FJ, Westenberg HGJ (2011) Electroconvulsive therapy has acute immunological and neuroendocrine effects in patients with major depressive disorder. Affect Disord 131:388–392. https://doi.org/10.1016/j.jad.2010.11.035
Bah TM, Benderdour M, Kaloustian S, Karam R, Rousseau G, Godbout R (2011) Escitalopram reduces circulating pro-inflammatory cytokines and improves depressive behavior without affecting sleep in a rat model of post-cardiac infarct depression. Behav Brain Res 225:243–251. https://doi.org/10.1016/j.bbr.2011.07.039
Munzer A, Sack U, Mergl R, Schönherr J, Petersein C, Bartsch S, Kirkby KC, Bauer K et al (2013) Impact of Antidepressants on Cytokine Production of Depressed Patients in Vitro. Toxins 5:2227–2240. https://doi.org/10.3390/toxins5112227
Li Y, Shen R, Wen G, Ding R, Du A, Zhou J, Dong Z, Ren X et al (2017) Effects of Ketamine on Levels of Inflammatory Cytokines IL-6, IL-1β, and TNF-α in the Hippocampus of Mice Following Acute or Chronic Administration. Front Pharmacol 8:139. https://doi.org/10.3389/fphar.2017.00139
Eller T, Vasar V, Shlik J, Maron E (2008) Pro-inflammatory cytokines and treatment response to escitalopram in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry 32:445–450. https://doi.org/10.1016/j.pnpbp.2007.09.015
Honack W, Loscher (1993) Sex differences in NMDA receptor mediated responses in rats. Brain Res 620:167–170. https://doi.org/10.1016/0006-8993(93)90287-w
Auer RN (1996) Effect of age and sex on N-methyl-D-aspartate antagonist-induced neuronal necrosis in rats. Stroke 27:743–746. https://doi.org/10.1161/01.str.27.4.743
Shors TJ, Falduto J, Leuner B (2004) The opposite effects of stress on dendritic spines in male vs. female rats are NMDA receptor-dependent. Eur J Neurosci 19:145–150. https://doi.org/10.1046/j.1460-9568.2003.03065.x
Yrondi A, Sporer M, Péran P, Schmitt L, Arbus C, Sauvaget A (2018) Electroconvulsive therapy, depression, the immune system and inflammation: A systematic review. Brain Stimul 11:29–51. https://doi.org/10.1016/j.brs.2017.10.013
Więdłocha M, Marcinowicz P, Krupa R, Janoska-Jaździk M, Janus M, Dębowska W, Mosiołek A, Waszkiewicz N et al (2018) Effect of antidepressant treatment on peripheral inflammation markers – a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry 80:217–226. https://doi.org/10.1016/j.pnpbp.2017.04.026
Réus GZ, Dos Santos MA, Abelaira HM, Ribeiro KF, Petronilho F, Vuolo F, Colpo GD, Pfaffenseller B et al (2013) Imipramine reverses alterations in cytokines and BDNF levels induced by maternal deprivation in adult rats. Behav Brain Res 242:40–46. https://doi.org/10.1016/j.bbr.2012.11.044
Dong C, Zhang J, Yao W, Ren Q, Yang C, Ma M, Han M, Saito R et al (2016) Effects of escitalopram, R-citalopram, and reboxetine on serum levels of tumor necrosis factor-α, interleukin-10, and depression-like behavior in mice after lipopolysaccharide administration. Pharmacol Biochem Behav 144:7–12. https://doi.org/10.1016/j.pbb.2016.02.005
Zhan Y, Zhou Y, Zheng W, Liu W, Wang C, Lan X, Deng X, Xu Y et al (2020) Alterations of multiple peripheral inflammatory cytokine levels after repeated ketamine infusions in major depressive disorder. Transl Psychiatry 10:246. https://doi.org/10.1038/s41398-020-00933-z
McCarthy MM (2016) Multifaceted origins of sex differences in the brain. Philos Trans R Soc Lond B Biol Sci 371:20150106. https://doi.org/10.1098/rstb.2015.0106
Acknowledgements
The Translational Psychiatry Program (USA) is funded by a grant from the National Institute of Health/National Institute of Mental Health (1R21MH117636-01A1, to JQ). The Center of Excellence on Mood Disorders (USA) is funded by the Pat Rutherford Jr. Chair in Psychiatry, John S. Dunn Foundation and Anne and Don Fizer Foundation Endowment for Depression Research. The Translational Psychiatry Laboratory (Brazil) is funded by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (GZR and JQ), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (JQ and GZR), Fundação de Amparo à Pesquisa e Inovação do Estado de Santa Catarina (FAPESC) (GZR and JQ), and Instituto Cérebro e Mente (JQ and GZR). JQ is a 1A and GZR is a 2 CNPq Research Fellow.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
All authors participated in the design and interpretation of the studies, analyzed the data and reviewed the manuscript; NSM conducted the maternal deprivation protocol. TR, MEMB, ABM, NMA, NSM, LRM, LAB and BCC performed drugs and ECT application, behavioral tests and sample collection. LGD, LJ, SB and FP performed the oxidative stress analysis. GZR and COA performed the statistical analysis. HMA, ABM and GZR wrote the manuscript. JQ and TT reviewed the manuscript. GZR and TR were in charge of designing the experiment and reviewed the manuscript.
Corresponding author
Ethics declarations
None.
Compliance with ethical standards
This study complies with ethical concerns and was approved by the UNESC's Ethics Committee under protocol number: 133–2019.
Consent to participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abelaira, H.M., Rosa, T., de Moura, A.B. et al. Combination of electroconvulsive stimulation with ketamine or escitalopram protects the brain against inflammation and oxidative stress induced by maternal deprivation and is critical for associated behaviors in male and female rats. Mol Neurobiol 59, 1452–1475 (2022). https://doi.org/10.1007/s12035-021-02718-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02718-x