Abstract
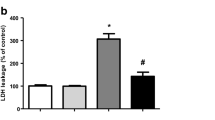
Tanshinone I (T-I; C18H12O3) is a cytoprotective molecule. T-I has been viewed as an antioxidant and anti-inflammatory agent exerting neuroprotective actions in several experimental models. Nonetheless, the mechanisms underlying the beneficial effects of T-I in mammalian cells are not completely understood yet. Mitochondrial dysfunction has been associated with several neurodegenerative diseases which remain uncured. Therefore, there is increasing interest in compounds that may be used in the prevention or treatment of those pathologies. Since T-I presents an antioxidant capacity, we investigated here whether and how this compound would prevent mitochondrial impairment in SH-SY5Y cells exposed to hydrogen peroxide (H2O2), which has been involved in the triggering of deleterious effects in several experimental models mimicking neurodegenerative processes. We found that a pretreatment with T-I at 2.5 μM for 2 h suppressed the pro-oxidant effects of H2O2 on mitochondrial membranes. Furthermore, T-I prevented the H2O2-elicited inhibition of the tricarboxylic acid (TCA) cycle enzymes (aconitase, α-ketoglutarate dehydrogenase, and succinate dehydrogenase) and of the mitochondrial complexes I and V. T-I also abrogated the mitochondrial depolarization and the mitochondrial failure to produce ATP in cells exposed to H2O2. T-I upregulated the levels of reduced glutathione (GSH) in the mitochondria of SH-SY5Y cells. T-I induced mitochondrial protection, at least in part, by activating the nuclear factor erythroid 2-related factor 2 (Nrf2), because silencing of Nrf2 by using small interference RNA (SiRNA) blocked these effects. Therefore, T-I afforded mitochondrial protection (involving both redox and bioenergetics-related aspects) against H2O2 through the activation of Nrf2.









Similar content being viewed by others
References
Tian XH, Wu JH (2013) Tanshinone derivatives: a patent review (January 2006 - September 2012). Expert Opin Ther Pat 23:19–29. doi:10.1517/13543776.2013.736494
de Oliveira MR, Schuck PF, Bosco SM (2016) Tanshinone I Induces Mitochondrial Protection through an Nrf2-Dependent Mechanism in Paraquat-TreatedHuman Neuroblastoma SH-SY5Y Cells. Mol Neurobiol doi. doi:10.1007/s12035-016-0009-x
Jing X, Wei X, Ren M, Wang L, Zhang X, Lou H (2016) Neuroprotective Effects of Tanshinone I Against 6-OHDA-Induced Oxidative Stress in Cellular and Mouse Model of Parkinson's Disease Through Upregulating Nrf2. Neurochem Res 41:779–786. doi:10.1007/s11064-015-1751-6
Zhang Y, Jiang P, Ye M, Kim SH, Jiang C, Lü J (2012) Tanshinones: sources, pharmacokinetics and anticancer activities. Int J Mol Sci 13:13621–13666. doi:10.3390/ijms131013621
Lee JC, Park JH, Park OK, Kim IH, Yan BC, Ahn JH, Kwon SH, Choi JH et al (2013) Neuroprotective effects of tanshinone I from Danshen extract in a mouse model of hypoxia-ischemia. Anat Cell Biol 46:183–190. doi:10.5115/acb.2013.46.3.183
Park JH, Ok P, Cho JH, Chen BH, Kim IH, Ahn JH, Lee JC, Yan BC et al (2014) Anti-inflammatory effect of tanshinone I in neuroprotection against cerebral ischemia-reperfusion injury in the gerbil hippocampus. Neurochem Res 39:1300–1312. doi:10.1007/s11064-014-1312-4
Park JH, Park OK, Yan B, Ahn JH, Kim IH, Lee JC, Kwon SH, Yoo KY et al (2014) Neuroprotection via maintenance or increase of antioxidants and neurotrophic factors in ischemic gerbil hippocampus treated with tanshinone I. Chin Med J 127:3396–3405
Wang S, Jing H, Yang H, Liu Z, Guo H, Chai L, Hu L (2015) Tanshinone I selectively suppresses proinflammatory genes expression in activated microglia and prevents nigrostriatal dopaminergic neurodegeneration in a mouse model of Parkinson's disease. J Ethnopharmacol 164:247–255. doi:10.1016/j.jep.2015.01.042
Rezin GT, Amboni G, Zugno AI, Quevedo J, Streck EL (2009) Mitochondrial dysfunction and psychiatric disorders. Neurochem Res 34:1021–1029. doi:10.1007/s11064-008-9865-8
Paradies G, Petrosillo G, Paradies V, Ruggiero FM (2011) Mitochondrial dysfunction in brain aging: role of oxidative stress and cardiolipin. Neurochem Int 58:447–457. doi:10.1016/j.neuint.2010.12.016
Bird MJ, Thorburn DR, Frazier AE (2014) Modelling biochemical features of mitochondrial neuropathology. Biochim Biophys Acta 1840:1380–1392. doi:10.1016/j.bbagen.2013.10.017
Kotiadis VN, Duchen MR, Osellame LD (2014) Mitochondrial quality control and communications with the nucleus are important in maintaining mitochondrial function and cell health. Biochim Biophys Acta 1840:1254–1265. doi:10.1016/j.bbagen.2013.10.041
Procaccini C, Santopaolo M, Faicchia D, Colamatteo A, Formisano L, de Candia P, Galgani M, De Rosa V et al (2016) Role of metabolism in neurodegenerative disorders. Metabolism 65:1376–1390. doi:10.1016/j.metabol.2016.05.018
Ryu SY, Peixoto PM, Teijido O, Dejean LM, Kinnally KW (2010) Role of mitochondrial ion channels in cell death. Biofactors 36:255–263. doi:10.1002/biof.101
Shoshan-Barmatz V, Ben-Hail D (2012) VDAC, a multi-functional mitochondrial protein as a pharmacological target. Mitochondrion 12:24–34. doi:10.1016/j.mito.2011.04.001
Green DR, Galluzzi L, Kroemer G (2014) Metabolic control of cell death. Science 345:1250256. doi:10.1126/science.1250256
Turrens JF (1997) Superoxide production by the mitochondrial respiratory chain. Biosci Rep 17:3–8
Lenaz G, Cavazzoni M, Genova ML, D'Aurelio M, Merlo Pich M, Pallotti F, Formiggini G, Marchetti M et al (1998) Oxidative stress, antioxidant defences and aging. Biofactors 8:195–204
Cadenas E, Davies KJ (2000) Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med 29:222–230
Naoi M, Maruyama W, Shamoto-Nagai M, Yi H, Akao Y, Tanaka M (2005) Oxidative stress in mitochondria: decision to survival and death of neurons in neurodegenerative disorders. Mol Neurobiol 31:81–93
Liu SS (2010) Mitochondrial Q cycle-derived superoxide and chemiosmotic bioenergetics. Ann N Y Acad Sci 1201:84–95. doi:10.1111/j.1749-6632.2010.05632.x
Brown GC (1992) Control of respiration and ATP synthesis in mammalian mitochondria and cells. Biochem J 284:1–13
Hill BG, Benavides GA, Lancaster JR Jr, Ballinger S, Dell'Italia L, Jianhua Z, Darley-Usmar VM (2012) Integration of cellular bioenergetics with mitochondrial quality control and autophagy. Biol Chem 393:1485–1512
Westermann B (2012) Bioenergetic role of mitochondrial fusion and fission. Biochim Biophys Acta 1817:1833–1838. doi:10.1016/j.bbabio.2012.02.033
Giorgi C, Agnoletto C, Bononi A, Bonora M, De Marchi E, Marchi S, Missiroli S, Patergnani S et al (2012) Mitochondrial calcium homeostasis as potential target for mitochondrial medicine. Mitochondrion 12:77–85. doi:10.1016/j.mito.2011.07.004
de Oliveira MR, Soares Oliveira MW, Müller Hoff ML, Behr GA, da Rocha RF, Fonseca Moreira JC (2009) Evaluation of redox and bioenergetics states in the liver of vitamin A-treated rats. Eur J Pharmacol 610:99–105. doi:10.1016/j.ejphar.2009.03.046
Gobe G, Crane D (2010) Mitochondria, reactive oxygen species and cadmium toxicity in the kidney. Toxicol Lett 198:49–55. doi:10.1016/j.toxlet.2010.04.013
Abarikwu SO, Pant AB, Farombi EO (2012) 4-Hydroxynonenal induces mitochondrial-mediated apoptosis and oxidative stress in SH-SY5Y human neuronal cells. Basic Clin Pharmacol Toxicol 110:441–448. doi:10.1111/j.1742-7843.2011.00834.x
de Oliveira MR, da Rocha RF, Pasquali MA, Moreira JC (2012) The effects of vitamin A supplementation for 3 months on adult rat nigrostriatal axis: increased monoamine oxidase enzyme activity, mitochondrial redox dysfunction, increased β-amyloid(1-40) peptide and TNF-α contents, and susceptibility of mitochondria to an in vitro H2O2 challenge. Brain Res Bull 87:432–444. doi:10.1016/j.brainresbull.2012.01.005
de Oliveira MR (2015) Vitamin A and Retinoids as Mitochondrial Toxicants. Oxid Med Cell Longev 2015:140267. doi:10.1155/2015/140267
de Oliveira MR (2016) Fluoxetine and the mitochondria: A review of the toxicological aspects. Toxicol Lett 258:185–191. doi:10.1016/j.toxlet.2016.07.001
de Oliveira MR, Jardim FR (2016) Cocaine and mitochondria-related signaling in the brain: A mechanistic view and future directions. Neurochem Int 92:58–66. doi:10.1016/j.neuint.2015.12.006
Atamna H, Mackey J, Dhahbi JM (2012) Mitochondrial pharmacology: electron transport chain bypass as strategies to treat mitochondrial dysfunction. Biofactors 38:158–166
Gruber J, Fong S, Chen CB, Yoong S, Pastorin G, Schaffer S, Cheah I, Halliwell B (2013) Mitochondriatargeted antioxidants and metabolic modulators as pharmacological interventions to slow ageing. Biotechnol Adv 31:563–592. doi:10.1016/j.biotechadv.2012.09.005
de Oliveira MR, Nabavi SF, Habtemariam S, Erdogan Orhan I, Daglia M, Nabavi SM (2015) The effects of baicalein and baicalin on mitochondrial function and dynamics: A review. Pharmacol Res 100:296–308. doi:10.1016/j.phrs.2015.08.021
de Oliveira MR, Jardim FR, Setzer WN, Nabavi SM, Nabavi SF (2016) Curcumin, mitochondrial biogenesis, and mitophagy: Exploring recent data and indicating future needs. Biotechnol Adv 34:813–826. doi:10.1016/j.biotechadv.2016.04.004
de Oliveira MR, Nabavi SM, Braidy N, Setzer WN, Ahmed T, Nabavi SF (2016) Quercetin and the mitochondria: A mechanistic view. Biotechnol Adv 34:532–549. doi:10.1016/j.biotechadv.2015.12.014
de Oliveira MR, Nabavi SF, Manayi A, Daglia M, Hajheydari Z, Nabavi SM (2016) Resveratrol and the mitochondria: From triggering the intrinsic apoptotic pathway to inducing mitochondrial biogenesis, a mechanistic view. Biochim Biophys Acta 1860:727–745. doi:10.1016/j.bbagen.2016.01.017
Nguyen T, Nioi P, Pickett CB (2009) The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem 284:13291–13295. doi:10.1074/jbc.R900010200
Uruno A, Motohashi H (2011) The Keap1-Nrf2 system as an in vivo sensor for electrophiles. Nitric Oxide 25:153–160. doi:10.1016/j.niox.2011.02.007
Surh YJ, Kundu JK, Na HK (2008) Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals. Planta Med 74:1526–1539. doi:10.1055/s-0028-1088302
de Oliveira MR, Schuck PF, Bosco SM (2016) Tanshinone I Induces Mitochondrial Protection through an Nrf2-Dependent Mechanism in Paraquat-TreatedHuman Neuroblastoma SH-SY5Y Cells. Mol Neurobiol IN PRESS doi. doi:10.1007/s12035-016-0009-x
de Oliveira MR, Ferreira GC, Schuck PF, Dal Bosco SM (2015) Role for the PI3K/Akt/Nrf2 signaling pathway in the protective effects of carnosic acid against methylglyoxal-induced neurotoxicity in SH-SY5Y neuroblastoma cells. Chem Biol Interact 242:396–406. doi:10.1016/j.cbi.2015.11.003
de Oliveira MR, Ferreira GC, Schuck PF (2016) Protective effect of carnosic acid against paraquatinduced redox impairment and mitochondrial dysfunction in SH-SY5Y cells: Role for PI3K/Akt/Nrf2 pathway. Toxicol In Vitro 32:41–54. doi:10.1016/j.tiv.2015.12.005
de Oliveira MR, Peres A, Ferreira GC, Schuck PF, Bosco SM (2016) Carnosic Acid Affords Mitochondrial Protection in Chlorpyrifos-Treated Sh-Sy5y Cells. Neurotox Res 30:367–379. doi:10.1007/s12640-016-9620-x
Haas R, Cucchi D, Smith J, Pucino V, Macdougall CE, Mauro C (2016) Intermediates of Metabolism: From Bystanders to Signalling Molecules. Trends Biochem Sci 41:460–471. doi:10.1016/j.tibs.2016.02.003
Tretter L, Adam-Vizi V (2000) Inhibition of Krebs cycle enzymes by hydrogen peroxide: A key role of [alpha]-ketoglutarate dehydrogenase in limiting NADH production under oxidative stress. J Neurosci 20:8972–8979
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
LeBel CP, Ischiropoulos H, Bondy SC (1992) Evaluation of the probe 2',7'-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol 5:227–231
De Oliveira MR, Oliveira MW, Da Rocha RF, Moreira JC (2009) Vitamin A supplementation at pharmacological doses induces nitrosative stress on the hypothalamus of adult Wistar rats. Chem Biol Interact 180:407–413. doi:10.1016/j.cbi.2009.02.006
de Oliveira MR, Lorenzi R, Schnorr CE, Morrone M, Moreira JC (2011) Increased 3-nitrotyrosine levels in mitochondrial membranes and impaired respiratory chain activity in brain regions of adult female rats submitted to daily vitamin A supplementation for 2 months. Brain Res Bull 86:246–253. doi:10.1016/j.brainresbull.2011.08.006
de Oliveira MR, da Rocha RF, Schnorr CE, Moreira JC (2012) L-NAME cotreatment did prevent neither mitochondrial impairment nor behavioral abnormalities in adult Wistar rats treated with vitamin A supplementation. Fundam Clin Pharmacol 26:513–529. doi:10.1111/j.1472-8206.2011.00943.x
Wang K, Zhu L, Zhu X, Zhang K, Huang B, Zhang J, Zhang Y, Zhu L et al (2014) Protective effect of paeoniflorin on Aβ25-35-induced SH-SY5Y cell injury by preventing mitochondrial dysfunction. Cell Mol Neurobiol 34:227–234. doi:10.1007/s10571-013-0006-9
Poderoso JJ, Carreras MC, Lisdero C, Riobó N, Schöpfer F, Boveris A (1996) Nitric oxide inhibits electron transfer and increases superoxide radical production in rat heart mitochondria and submitochondrial particles. Arch Biochem Biophys 328:85–92
De Oliveira MR, Moreira JC (2008) Impaired redox state and respiratory chain enzyme activities in the cerebellum of vitamin A-treated rats. Toxicology 253:125–130. doi:10.1016/j.tox.2008.09.003
Atamna H, Frey WH 2nd (2007) Mechanisms of mitochondrial dysfunction and energy deficiency in Alzheimer's disease. Mitochondrion 7:297–310
Bird MJ, Thorburn DR, Frazier AE (2014) Modelling biochemical features of mitochondrial neuropathology. Biochim Biophys Acta 1840:1380–1392. doi:10.1016/j.bbagen.2013.10.017
Veyrat-Durebex C, Corcia P, Piver E, Devos D, Dangoumau A, Gouel F, Vourc'h P, Emond P et al (2015) Disruption of TCA Cycle and Glutamate Metabolism Identified by Metabolomics in an In Vitro Model of Amyotrophic Lateral Sclerosis. Mol Neurobiol IN PRESS. doi:10.1007/s12035-015-9567-6
Long Y, Dong X, Yuan Y, Huang J, Song J, Sun Y, Lu Z, Yang L et al (2015) Metabolomics changes in a rat model of obstructive jaundice: mapping to metabolism of amino acids, carbohydrates and lipids as well as oxidative stress. J Clin Biochem Nutr 57:50–59. doi:10.3164/jcbn.14-147
Macongonde EA, Vilela TC, Scaini G, Gonçalves CL, Ferreira BK, Costa NL, de Oliveira MR, Avila Junior S et al (2015) Evaluation of the In Vivo and In Vitro Effects of Fructose on Respiratory Chain Complexes in Tissues of Young Rats. Dis Markers 2015:312530. doi:10.1155/2015/312530
Schuck PF, Malgarin F, Cararo JH, Cardoso F, Streck EL, Ferreira GC (2015) Phenylketonuria Pathophysiology: on the Role of Metabolic Alterations. Aging Dis 6:390–399. doi:10.14336/AD.2015.0827
Zandberg L, van Dyk HC, van der Westhuizen FH, van Dijk AA (2016) A 3-methylcrotonyl-CoA carboxylase deficient human skin fibroblast transcriptome reveals underlying mitochondrial dysfunction and oxidative stress. Int J Biochem Cell Biol 78:116–129. doi:10.1016/j.biocel.2016.07.010
Uttara B, Singh AV, Zamboni P, Mahajan RT (2009) Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol 7:65–74. doi:10.2174/157015909787602823
Szewczyk A, Wojtczak L (2002) Mitochondria as a pharmacological target. Pharmacol Rev 54:101–127
Smith RA, Hartley RC, Cochemé HM, Murphy MP (2012) Mitochondrial pharmacology. Trends Pharmacol Sci 33:341–352. doi:10.1016/j.tips.2012.03.010
Oliveira MR, Nabavi SF, Daglia M, Rastrelli L, Nabavi SM (2016) Epigallocatechin gallate and mitochondria-A story of life and death. Pharmacol Res 104:70–85. doi:10.1016/j.phrs.2015.12.027
de Oliveira MR (2016) Evidence for genistein as a mitochondriotropic molecule. Mitochondrion 29:35–44. doi:10.1016/j.mito.2016.05.005
de Oliveira MR, Silvestrin RB, Mello E, Souza T, Moreira JC (2007) Oxidative stress in the hippocampus, anxiety-like behavior and decreased locomotory and exploratory activity of adult rats: effects of sub acute vitamin A supplementation at therapeutic doses. Neurotoxicology 28:1191–1199
De Oliveira MR, Oliveira MW, Behr GA, Moreira JC (2009) Vitamin A supplementation at clinical doses induces a dysfunction in the redox and bioenergetics states, but did change neither caspases activities nor TNF-alpha levels in the frontal cortex of adult Wistar rats. J Psychiatr Res 43:754–762. doi:10.1016/j.jpsychires.2008.10.002
Navarro A, Boveris A (2008) Mitochondrial nitric oxide synthase, mitochondrial brain dysfunction in aging, and mitochondria-targeted antioxidants. Adv Drug Deliv Rev 60:1534–1544. doi:10.1016/j.addr.2008.05.002
Yin F, Boveris A, Cadenas E (2014) Mitochondrial energy metabolism and redox signaling in brain aging and neurodegeneration. Antioxid Redox Signal 20:353–371. doi:10.1089/ars.2012.4774
Kussmaul L, Hirst J (2006) The mechanism of superoxide production by NADH:ubiquinone oxidoreductase (complex I) from bovine heart mitochondria. Proc Natl Acad Sci U S A 103:7607–7612
Grivennikova VG, Vinogradov AD (2006) Generation of superoxide by the mitochondrial Complex I. Biochim Biophys Acta 1757:553–561
Pryde KR, Hirst J (2011) Superoxide is produced by the reduced flavin in mitochondrial complex I: a single, unified mechanism that applies during both forward and reverse electron transfer. J Biol Chem 286:18056–18065. doi:10.1074/jbc.M110.186841
Giachin G, Bouverot R, Acajjaoui S, Pantalone S, Soler-López M (2016) Dynamics of Human Mitochondrial Complex I Assembly: Implications for Neurodegenerative Diseases. Front Mol Biosci 3:43. doi:10.3389/fmolb.2016.00043
Loots DT (2009) Abnormal tricarboxylic acid cycle metabolites in isovaleric acidaemia. J Inherit Metab Dis 32:403–411. doi:10.1007/s10545-009-1071-6
Gray LR, Tompkins SC, Taylor EB (2014) Regulation of pyruvate metabolism and human disease. Cell Mol Life Sci 71:2577–2604. doi:10.1007/s00018-013-1539-2
Candas D, Li JJ (2014) MnSOD in oxidative stress response-potential regulation via mitochondrial protein influx. Antioxid Redox Signal 20:1599–1617. doi:10.1089/ars.2013.5305
Lei XG (2001) Glutathione peroxidase-1 gene knockout on body antioxidant defense in mice. Biofactors 14:93–99
Brigelius-Flohé R, Kipp A (2009) Glutathione peroxidases in different stages of carcinogenesis. Biochim Biophys Acta 1790:1555–1568. doi:10.1016/j.bbagen.2009.03.006
Brigelius-Flohé R, Maiorino M (2013) Glutathione peroxidases. Biochim Biophys Acta 1830:3289–3303. doi:10.1016/j.bbagen.2012.11.020
Lu SC (2013) Glutathione synthesis. Biochim Biophys Acta 1830:3143–3153. doi:10.1016/j.bbagen.2012.09.008
Dinkova-Kostova AT, Abramov AY (2015) The emerging role of Nrf2 in mitochondrial function. Free Radic Biol Med 88:179–188. doi:10.1016/j.freeradbiomed.2015.04.036
Acknowledgments
GCF is supported by Edital APQ1/FAPERJ and receives a “ Produtividade em Pesquisa do CNPq - Nível 2” fellow. ICCS received a MCTI/CNPq/Universal 14/2014 fellow. CRF is supported by Edital MCTI/CNPQ/Universal 14/2014 – Processo 446747/2014-9. This work was supported by CNPq.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
None to declare.
Electronic supplementary material
Figure S1.
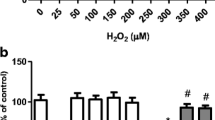
The effect of different concentrations of H2O2 for 24 h on the viability of SH-SY5Y cells. Data are shown as the mean ± S.E.M. of three or five independent experiments each done in triplicate. One-way ANOVA followed by the post hoc Tukey’s test, * p < 0.05 vs control cells (PDF 96 kb)
Figure S2.
The effects of a pretreatment (2 h) with 1–5 μM tanshinone-I (T-I) on the the viability of SH-SY5Y cells. Data are shown as the mean ± SEM of three or five independent experiments each done in triplicate. One-way ANOVA followed by the post hoc Tukey’s test, *p < 0.05 vs the control group, # p < 0.05 different from H2O2-treated cells (PDF 96 kb)
Rights and permissions
About this article
Cite this article
de Oliveira, M.R., Fürstenau, C.R., de Souza, I.C.C. et al. Tanshinone I Attenuates the Effects of a Challenge with H2O2 on the Functions of Tricarboxylic Acid Cycle and Respiratory Chain in SH-SY5Y Cells. Mol Neurobiol 54, 7858–7868 (2017). https://doi.org/10.1007/s12035-016-0267-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-0267-7