Abstract
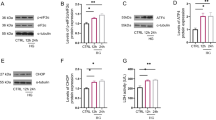
Acetylcholinesterase (AChE) is impaired in brain of diabetic animals, which may be one of the reasons for diabetes-associated cognitive decline. However, the mechanism is still unknown. The present study was designed to investigate whether the increased expression of AChE in central neurons under high glucose (HG) condition was due to activation of mammalian target of rapamycin (mTOR) signaling. It was found that more production of reactive oxygen species, and higher levels of phospho-Akt, phospho-mTOR, phospho-p70S6K, and AChE were detected in HT-22 cells in HG group than normal glucose group after culture for 24 h, which were all attenuated by an antioxidant N-acetyl-l-cysteine. A PI3K inhibitor LY294002 significantly decreased the levels of phospho-Akt, phospho-mTOR, phospho-p70S6K, and AChE protein expression in HG-cultured HT-22 cells, and an mTOR inhibitor rapamycin markedly reduced the levels of phospho-mTOR, phospho-p70S6K, and AChE expression. Furthermore, compared with normal rats, diabetic rats showed remarkable increases in levels of AChE activity and expression, malondialdehyde, phospho-mTOR, phospho-p70S6K, and a significant decrease in total superoxide dismutase activity in both hippocampus and cerebral cortex. However, much lower levels of phospho-mTOR, phospho-p70S6K, and AChE expression occurred in both brain regions of diabetic rats treated with rapamycin when compared with untreated ones. These results indicated that mTOR signaling was activated through the activation of PI3K/Akt pathway mediated by oxidative stress in HG-cultured HT-22 cells and diabetic rat brains, which contributed to the elevated protein expression of AChE in central neurons under the condition of HG.







Similar content being viewed by others
Abbreviations
- AChE:
-
Acetylcholinesterase
- Cont:
-
Control
- DACD:
-
Diabetes-associated cognitive decline
- DCFH-DA:
-
2′,7′-Dichlorofluorescein diacetate
- DM:
-
Diabetes mellitus
- DMSO:
-
Dimethylsulfoxide
- HG:
-
High glucose
- HPLC:
-
High-performance liquid chromatography
- LY:
-
LY294002
- MDA:
-
Malondialdehyde
- mTOR:
-
Mammalian target of rapamycin
- NAC:
-
N-Acetyl-l-cysteine
- NG:
-
Normal glucose
- p70S6K:
-
p70 ribosomal S6 protein kinase
- PI3K:
-
Phosphatidylinositol 3 kinase
- Rap:
-
Rapamycin
- ROS:
-
Reactive oxygen species
- STZ:
-
Streptozotocin
- T-SOD:
-
Total superoxidase dismutase
References
Brands AM et al (2005) The effects of type 1 diabetes on cognitive performance: a meta-analysis. Diabetes Care 28:726–735
Mijnhout GS et al (2006) Diabetic encephalopathy: a concept in need of a definition. Diabetologia 49:1447–1448
Manschot SM et al (2003) Angiotensin converting enzyme inhibition partially prevents deficits in water maze performance, hippocampal synaptic plasticity and cerebral blood flow in streptozotocin-diabetic rats. Brain Res 966:274–282
Kuhad A, Chopra K (2007) Curcumin attenuates diabetic encephalopathy in rats: behavioral and biochemical evidences. Eur J Pharmacol 576:34–42
Saxena G et al (2007) Gugulipid, an extract of Commiphora whighitii with lipid-lowering properties, has protective effects against streptozotocin-induced memory deficits in mice. Pharmacol Biochem Behav 86:797–805
Kuhad A et al (2009) Suppression of NF-kappabeta signaling pathway by tocotrienol can prevent diabetes associated cognitive deficits. Pharmacol Biochem Behav 92:251–259
Liu YW et al (2012) Total saponins from Rhizoma Anemarrhenae ameliorate diabetes-associated cognitive decline in rats: involvement of amyloid-beta decrease in brain. J Ethnopharmacol 139:194–200
van Deutekom AW et al (2008) Increased Nepsilon-(carboxymethyl)-lysine levels in cerebral blood vessels of diabetic patients and in a (streptozotocin-treated) rat model of diabetes mellitus. Eur J Endocrinol 158:655–660
Wang SH et al (2009) Diabetes impairs hippocampal function via advanced glycation end product mediated new neuron generation in animals with diabetes-related depression. Toxicol Sci 111:72–79
Li ZG et al (2005) The role of impaired insulin/IGF action in primary diabetic encephalopathy. Brain Res 1037:12–24
Lakhman SS, Kaur G (1994) Effect of alloxan-induced diabetes on acetylcholinesterase activity from discrete areas of rat brain. Neurochem Int 24:159–163
Bhutada P et al (2011) Protection of cholinergic and antioxidant system contributes to the effect of berberine ameliorating memory dysfunction in rat model of streptozotocin-induced diabetes. Behav Brain Res 220:30–41
Schmatz R et al (2009) Resveratrol prevents memory deficits and the increase in acetylcholinesterase activity in streptozotocin-induced diabetic rats. Eur J Pharmacol 610:42–48
Bhutada P et al (2010) Ameliorative effect of quercetin on memory dysfunction in streptozotocin-induced diabetic rats. Neurobiol Learn Mem 94:293–302
Zhao Q et al (2011) Diabetes-induced central cholinergic neuronal loss and cognitive deficit are attenuated by tacrine and a Chinese herbal prescription, kangen-karyu: elucidation in type 2 diabetes db/db mice. J Pharmacol Sci 117:230–242
Brownlee M (2005) The pathobiology of diabetic complications: a unifying mechanism. Diabetes 54:1615–1625
Hasanein P, Shahidi S (2010) Effects of combined treatment with vitamins C and E on passive avoidance learning and memory in diabetic rats. Neurobiol Learn Mem 93:472–478
Hay N, Sonenberg N (2004) Upstream and downstream of mTOR. Genes Dev 18:1926–1945
Martin DE, Hall MN (2005) The expanding TOR signaling network. Curr Opin Cell Biol 17:158–166
Burnett PE et al (1998) RAFT1 phosphorylation of the translational regulators p70 S6 kinase and 4E-BP1. Proc Natl Acad Sci U S A 95:1432–1437
Richter JD, Klann E (2009) Making synaptic plasticity and memory last: mechanisms of translational regulation. Genes Dev 23:1–11
Puighermanal E et al (2009) Cannabinoid modulation of hippocampal long-term memory is mediated by mTOR signaling. Nat Neurosci 12:1152–1158
Tischmeyer W et al (2003) Rapamycin-sensitive signalling in long-term consolidation of auditory cortex-dependent memory. Eur J Neurosci 18:942–950
Ehninger D et al (2009) From mTOR to cognition: molecular and cellular mechanisms of cognitive impairments in tuberous sclerosis. J Intellect Disabil Res 53:838–851
Faghiri Z, Bazan NG (2010) PI3K/Akt and mTOR/p70S6K pathways mediate neuroprotectin D1-induced retinal pigment epithelial cell survival during oxidative stress-induced apoptosis. Exp Eye Res 90:718–725
Chong ZZ, Maiese K (2012) Mammalian target of rapamycin signaling in diabetic cardiovascular disease. Cardiovasc Diabetol 11:45
Lloberas N et al (2006) Mammalian target of rapamycin pathway blockade slows progression of diabetic kidney disease in rats. J Am Soc Nephrol 17:1395–1404
Mori H et al (2009) The mTOR pathway is highly activated in diabetic nephropathy and rapamycin has a strong therapeutic potential. Biochem Biophys Res Commun 384:471–475
Yang Y et al (2007) Rapamycin prevents early steps of the development of diabetic nephropathy in rats. Am J Nephrol 27:495–502
Davis JB, Maher P (1994) Protein kinase C activation inhibits glutamate-induced cytotoxicity in a neuronal cell line. Brain Res 652:169–173
Liu D et al (2014) Effects of exposure to high glucose on primary cultured hippocampal neurons: involvement of intracellular ROS accumulation. Neurol Sci 35:831–837
Pamidi N, Satheesha Nayak BN (2012) Effect of streptozotocin induced diabetes on rat hippocampus. Bratisl Lek Listy 113:583–588
Chen X et al (2012) Tacrine-silibinin codrug shows neuro- and hepatoprotective effects in vitro and pro-cognitive and hepatoprotective effects in vivo. J Med Chem 55:5231–5242
Fukui M et al (2010) Mechanism for the protective effect of resveratrol against oxidative stress-induced neuronal death. Free Radic Biol Med 49:800–813
Tan S et al (1998) Oxidative stress induces a form of programmed cell death with characteristics of both apoptosis and necrosis in neuronal cells. J Neurochem 71:95–105
Zhang X et al (2013) Endoplasmic reticulum stress-mediated hippocampal neuron apoptosis involved in diabetic cognitive impairment. Biomed Res Int 2013:924327
Sharifi AM et al (2007) Study of high glucose-induced apoptosis in PC12 cells: role of bax protein. J Pharmacol Sci 104:258–262
Kumar P et al (2014) Hyperglycemia-induced oxidative stress induces apoptosis by inhibiting PI3-kinase/Akt and ERK1/2 MAPK mediated signaling pathway causing downregulation of 8-oxoG-DNA glycosylase levels in glial cells. Int J Biochem Cell Biol 53:302–319
Memmott RM, Dennis PA (2009) Akt-dependent and -independent mechanisms of mTOR regulation in cancer. Cell Signal 21:656–664
Garelick MG, Kennedy BK (2011) TOR on the brain. Exp Gerontol 46:155–163
Ma YQ et al (2013) mTOR and tau phosphorylated proteins in the hippocampal tissue of rats with type 2 diabetes and Alzheimer’s disease. Mol Med Rep 7:623–627
Acknowledgments
The work was supported through funding from the Natural Science Foundation of China (81371210) and Qing Lan Project, China, and a project funded through the Priority Academic Program Development of Jiangsu Higher Education Institutions, China.
Conflict of Interest
The authors have declared that there is no conflict of interest.
Compliance with Ethical Standards
All animal experiments were approved through the Animal Ethics Committee of Xuzhou Medical College. All experiments were performed according to the Guidelines for Ethical Conduct in the Care and Use of Animals.
Author information
Authors and Affiliations
Corresponding author
Additional information
Yao-Wu Liu and Liang Zhang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Liu, YW., Zhang, L., Li, Y. et al. Activation of mTOR signaling mediates the increased expression of AChE in high glucose condition: in vitro and in vivo evidences. Mol Neurobiol 53, 4972–4980 (2016). https://doi.org/10.1007/s12035-015-9425-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-015-9425-6