Abstract
The adult midbrain contains 75 % of all dopaminergic neurons in the CNS. Within the midbrain, these neurons are divided into three anatomically and functionally distinct clusters termed A8, A9 and A10. The A9 group plays a functionally non-redundant role in the control of voluntary movement, which is highlighted by the motor syndrome that results from their progressive degeneration in the neurodegenerative disorder, Parkinson’s disease. Despite 50 years of investigation, treatment for Parkinson’s disease remains symptomatic, but an intensive research effort has proposed delivering neurotrophic factors to the brain to protect the remaining dopaminergic neurons, or using these neurotrophic factors to differentiate dopaminergic neurons from stem cell sources for cell transplantation. Most neurotrophic factors studied in this context have been members of the transforming growth factor β (TGFβ) superfamily. In recent years, an intensive research effort has focused on understanding the function of these proteins in midbrain dopaminergic neuron development and their role in the molecular architecture that regulates the development of this brain region, with the goal of applying this knowledge to develop novel therapies for Parkinson’s disease. In this review, the current evidence showing that TGFβ superfamily members play critical roles in the regulation of midbrain dopaminergic neuron induction, differentiation, target innervation and survival during embryonic and postnatal development is analysed, and the implications of these findings are discussed.
Similar content being viewed by others
Abbreviations
- 6-OHDA:
-
6-Hydroxydopamine
- ALK:
-
Activin receptor-like kinases
- BDNF:
-
Brain-derived neurotrophic factor
- BMP:
-
Bone morphogenetic protein
- BMPR:
-
BMP receptors
- Co-Smads:
-
Common mediator Smads
- DA:
-
Dopaminergic/dopamine
- E:
-
Embryonic day
- FGF:
-
Fibroblast growth factor
- GDF:
-
Growth/differentiation factor
- GDNF:
-
Glial cell line-derived neurotrophic factor
- I-Smads:
-
Inhibitory Smads
- MPP+:
-
1-Methyl-4-phenylpyridinium ion
- P:
-
Postnatal day
- PD:
-
Parkinson’s disease
- R-Smads:
-
Receptor-regulated Smads
- Shh:
-
Sonic hedgehog
- TGFβ:
-
Transforming growth factor β
- TH:
-
Tyrosine hydroxylase
- VM:
-
Ventral midbrain/mesencephalon
References
Ono Y, Nakatani T, Sakamoto Y, Mizuhara E, Minaki Y, Kumai M, Hamaguchi A, Nishimura M, Inoue Y, Hayashi H, Takahashi J, Imai T (2007) Differences in neurogenic potential in floor plate cells along an anteroposterior location: midbrain dopaminergic neurons originate from mesencephalic floor plate cells. Development 134(17):3213–3225
Dahlstroem A, Fuxe K (1964) Evidence for the existence of monoamine-containing neurons in the central nervous system. I. demonstration of monoamines in the cell bodies of brain stem neurons. Acta Physiol Scand Suppl 232:231–255
Bjorklund A, Dunnett SB (2007) Dopamine neuron systems in the brain: an update. Trends Neurosci 30(5):194–202
Toulouse A, Sullivan AM (2008) Progress in Parkinson’s disease—where do we stand? Prog Neurobiol 85(4):376–392
Lees AJ, Hardy J, Revesz T (2009) Parkinson’s disease. Lancet 373(9680):2055–2066
Meyer-Lindenberg A, Miletich RS, Kohn PD, Esposito G, Carson RE, Quarantelli M, Weinberger DR, Berman KF (2002) Reduced prefrontal activity predicts exaggerated striatal dopaminergic function in schizophrenia. Nat Neurosci 5(3):267–271
Robinson TE, Berridge KC (1993) The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev 18(3):247–291
de Lau LM, Breteler MM (2006) Epidemiology of Parkinson’s disease. Lancet Neurol 5(6):525–535
Sullivan AM, Toulouse A (2011) Neurotrophic factors for the treatment of Parkinson’s disease. Cytokine Growth Factor Rev 22(3):157–165
Peterson AL, Nutt JG (2008) Treatment of Parkinson's disease with trophic factors. Neurotherapeutics 5(2):270–280
Bottner M, Krieglstein K, Unsicker K (2000) The transforming growth factor-betas: structure, signaling, and roles in nervous system development and functions. J Neurochem 75(6):2227–2240
Sullivan AM, O’Keeffe GW (2005) The role of growth/differentiation factor 5 (GDF5) in the induction and survival of midbrain dopaminergic neurones: relevance to Parkinson’s disease treatment. J Anat 207(3):219–226
Massague J, Wotton D (2000) Transcriptional control by the TGF-beta/Smad signaling system. EMBO J 19(8):1745–1754
Yamashita H, Ten Dijke P, Heldin CH, Miyazono K (1996) Bone morphogenetic protein receptors. Bone 19(6):569–574
Shi Y, Massague J (2003) Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 113(6):685–700
Nohe A, Keating E, Knaus P, Petersen NO (2004) Signal transduction of bone morphogenetic protein receptors. Cell Signal 16(3):291–299
Bragdon B, Moseychuk O, Saldanha S, King D, Julian J, Nohe A (2011) Bone morphogenetic proteins: a critical review. Cell Signal 23(4):609–620
Sieber C, Kopf J, Hiepen C, Knaus P (2009) Recent advances in BMP receptor signaling. Cytokine Growth Factor Rev 20(5–6):343–355
Miyazono K, Kusanagi K, Inoue H (2001) Divergence and convergence of TGF-beta/BMP signaling. J Cell Physiol 187(3):265–276
Massague J (2012) TGFbeta signalling in context. Nat Rev Mol Cell Biol 13(10):616–630
Moustakas A, Souchelnytskyi S, Heldin CH (2001) Smad regulation in TGF-beta signal transduction. J Cell Sci 114(Pt 24):4359–4369
Goumans MJ, Lebrin F, Valdimarsdottir G (2003) Controlling the angiogenic switch: a balance between two distinct TGF-b receptor signaling pathways. Trends Cardiovasc Med 13(7):301–307
Itoh S, Itoh F, Goumans MJ, Ten Dijke P (2000) Signaling of transforming growth factor-beta family members through Smad proteins. Eur J Biochem 267(24):6954–6967
Miyazawa K, Shinozaki M, Hara T, Furuya T, Miyazono K (2002) Two major Smad pathways in TGF-beta superfamily signalling. Genes Cells 7(12):1191–1204
Miyazono K, Maeda S, Imamura T (2005) BMP receptor signaling: transcriptional targets, regulation of signals, and signaling cross-talk. Cytokine Growth Factor Rev 16(3):251–263
Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F (1993) GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science 260(5111):1130–1132
Krieglstein K, Suter-Crazzolara C, Fischer WH, Unsicker K (1995) TGF-beta superfamily members promote survival of midbrain dopaminergic neurons and protect them against MPP + toxicity. EMBO J 14(4):736–742
Widmer HR, Schaller B, Meyer M, Seiler RW (2000) Glial cell line-derived neurotrophic factor stimulates the morphological differentiation of cultured ventral mesencephalic calbindin- and calretinin-expressing neurons. Exp Neurol 164(1):71–81
Clarkson ED, Zawada WM, Freed CR (1995) GDNF reduces apoptosis in dopaminergic neurons in vitro. Neuroreport 7(1):145–149
Clarkson ED, Zawada WM, Freed CR (1997) GDNF improves survival and reduces apoptosis in human embryonic dopaminergic neurons in vitro. Cell Tissue Res 289(2):207–210
Sawada H, Ibi M, Kihara T, Urushitani M, Nakanishi M, Akaike A, Shimohama S (2000) Neuroprotective mechanism of glial cell line-derived neurotrophic factor in mesencephalic neurons. J Neurochem 74(3):1175–1184
Orme RP, Bhangal MS, Fricker RA (2013) Calcitriol imparts neuroprotection in vitro to midbrain dopaminergic neurons by upregulating GDNF expression. PLoS One 8(4):e62040
Borgal L, Hong M, Sadi D, Mendez I (2007) Differential effects of glial cell line-derived neurotrophic factor on A9 and A10 dopamine neuron survival in vitro. Neuroscience 147(3):712–719
Apostolides C, Sanford E, Hong M, Mendez I (1998) Glial cell line-derived neurotrophic factor improves intrastriatal graft survival of stored dopaminergic cells. Neuroscience 83(2):363–372
Espejo M, Cutillas B, Arenas TE, Ambrosio S (2000) Increased survival of dopaminergic neurons in striatal grafts of fetal ventral mesencephalic cells exposed to neurotrophin-3 or glial cell line-derived neurotrophic factor. Cell Transplant 9(1):45–53
Granholm AC, Mott JL, Bowenkamp K, Eken S, Henry S, Hoffer BJ, Lapchak PA, Palmer MR, van Horne C, Gerhardt GA (1997) Glial cell line-derived neurotrophic factor improves survival of ventral mesencephalic grafts to the 6-hydroxydopamine lesioned striatum. Exp Brain Res 116(1):29–38
Yurek DM (1998) Glial cell line-derived neurotrophic factor improves survival of dopaminergic neurons in transplants of fetal ventral mesencephalic tissue. Exp Neurol 153(2):195–202
Sullivan AM, Pohl J, Blunt SB (1998) Growth/differentiation factor 5 and glial cell line-derived neurotrophic factor enhance survival and function of dopaminergic grafts in a rat model of Parkinson's disease. Eur J Neurosci 10(12):3681–3688
Burke RE (2003) Postnatal developmental programmed cell death in dopamine neurons. Ann N Y Acad Sci 991:69–79
Kholodilov N, Yarygina O, Oo TF, Zhang H, Sulzer D, Dauer W, Burke RE (2004) Regulation of the development of mesencephalic dopaminergic systems by the selective expression of glial cell line-derived neurotrophic factor in their targets. J Neurosci 24(12):3136–3146
Granholm AC, Reyland M, Albeck D, Sanders L, Gerhardt G, Hoernig G, Shen L, Westphal H, Hoffer B (2000) Glial cell line-derived neurotrophic factor is essential for postnatal survival of midbrain dopamine neurons. J Neurosci 20(9):3182–3190
Choi-Lundberg DL, Bohn MC (1995) Ontogeny and distribution of glial cell line-derived neurotrophic factor (GDNF) mRNA in rat. Brain Res Dev Brain Res 85(1):80–88
Gavin AM, Walsh S, Wyatt SL, O’Keeffe GW, Sullivan AM (2013) 6-Hydroxydopamine induces distinct alterations in GDF5 and GDNF mRNA expression in the rat nigrostriatal system in vivo. Neurosci Lett 561:176–181
Ahmadiantehrani S, Ron D (2013) Dopamine D2 receptor activation leads to an up-regulation of glial cell line-derived neurotrophic factor via Gbetagamma-Erk1/2-dependent induction of Zif268. J Neurochem 125(2):193–204
Eggert K, Schlegel J, Oertel W, Wurz C, Krieg JC, Vedder H (1999) Glial cell line-derived neurotrophic factor protects dopaminergic neurons from 6-hydroxydopamine toxicity in vitro. Neurosci Lett 269(3):178–182
Hou JG, Lin LF, Mytilineou C (1996) Glial cell line-derived neurotrophic factor exerts neurotrophic effects on dopaminergic neurons in vitro and promotes their survival and regrowth after damage by 1-methyl-4-phenylpyridinium. J Neurochem 66(1):74–82
Tomac A, Lindqvist E, Lin LF, Ogren SO, Young D, Hoffer BJ, Olson L (1995) Protection and repair of the nigrostriatal dopaminergic system by GDNF in vivo. Nature 373(6512):335–339
Gash DM, Zhang Z, Ovadia A, Cass WA, Yi A, Simmerman L, Russell D, Martin D, Lapchak PA, Collins F, Hoffer BJ, Gerhardt GA (1996) Functional recovery in parkinsonian monkeys treated with GDNF. Nature 380(6571):252–255
Connor B, Kozlowski DA, Unnerstall JR, Elsworth JD, Tillerson JL, Schallert T, Bohn MC (2001) Glial cell line-derived neurotrophic factor (GDNF) gene delivery protects dopaminergic terminals from degeneration. Exp Neurol 169(1):83–95
Kozlowski DA, Connor B, Tillerson JL, Schallert T, Bohn MC (2000) Delivery of a GDNF gene into the substantia nigra after a progressive 6-OHDA lesion maintains functional nigrostriatal connections. Exp Neurol 166(1):1–15
Date I, Aoi M, Tomita S, Collins F, Ohmoto T (1998) GDNF administration induces recovery of the nigrostriatal dopaminergic system both in young and aged parkinsonian mice. Neuroreport 9(10):2365–2369
Kordower JH, Emborg ME, Bloch J, Ma SY, Chu Y, Leventhal L, McBride J, Chen EY, Palfi S, Roitberg BZ, Brown WD, Holden JE, Pyzalski R, Taylor MD, Carvey P, Ling Z, Trono D, Hantraye P, Deglon N, Aebischer P (2000) Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson’s disease. Science 290(5492):767–773
Fox CM, Gash DM, Smoot MK, Cass WA (2001) Neuroprotective effects of GDNF against 6-OHDA in young and aged rats. Brain Res 896(1–2):56–63
Van den Heuvel DM, Pasterkamp RJ (2008) Getting connected in the dopamine system. Prog Neurobiol 85(1):75–93
Gill SS, Patel NK, Hotton GR, O’Sullivan K, McCarter R, Bunnage M, Brooks DJ, Svendsen CN, Heywood P (2003) Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat Med 9(5):589–595
Slevin JT, Gerhardt GA, Smith CD, Gash DM, Kryscio R, Young B (2005) Improvement of bilateral motor functions in patients with Parkinson disease through the unilateral intraputaminal infusion of glial cell line-derived neurotrophic factor. J Neurosurg 102(2):216–222
Lang AE, Gill S, Patel NK, Lozano A, Nutt JG, Penn R, Brooks DJ, Hotton G, Moro E, Heywood P, Brodsky MA, Burchiel K, Kelly P, Dalvi A, Scott B, Stacy M, Turner D, Wooten VG, Elias WJ, Laws ER, Dhawan V, Stoessl AJ, Matcham J, Coffey RJ, Traub M (2006) Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson disease. Ann Neurol 59(3):459–466
Xing B, Xin T, Zhao L, Hunter RL, Chen Y, Bing G (2010) Glial cell line-derived neurotrophic factor protects midbrain dopaminergic neurons against lipopolysaccharide neurotoxicity. J Neuroimmunol 225(1–2):43–51
Collins LM, Toulouse A, Connor TJ, Nolan YM (2012) Contributions of central and systemic inflammation to the pathophysiology of Parkinson’s disease. Neuropharmacology 62(7):2154–2168
Nolan YM, Sullivan AM, Toulouse A (2013) Parkinson’s disease in the nuclear age of neuroinflammation. Trends Mol Med 19(3):187–196
Hirsch EC, Vyas S, Hunot S (2012) Neuroinflammation in Parkinson’s disease. Parkinsonism Relat Disord 18(Suppl 1):S210–S212
Hegarty SV, Sullivan AM, O’Keeffe GW (2013) Midbrain dopaminergic neurons: a review of the molecular circuitry that regulates their development. Dev Biol 379(2):123–138
Lei Z, Jiang Y, Li T, Zhu J, Zeng S (2011) Signaling of glial cell line-derived neurotrophic factor and its receptor GFRalpha1 induce Nurr1 and Pitx3 to promote survival of grafted midbrain-derived neural stem cells in a rat model of Parkinson disease. J Neuropathol Exp Neurol 70(9):736–747
Roussa E, Krieglstein K (2004) GDNF promotes neuronal differentiation and dopaminergic development of mouse mesencephalic neurospheres. Neurosci Lett 361(1–3):52–55
Peng C, Aron L, Klein R, Li M, Wurst W, Prakash N, Le W (2011) Pitx3 is a critical mediator of GDNF-induced BDNF expression in nigrostriatal dopaminergic neurons. J Neurosci 31(36):12802–12815
Smidt MP, Smits SM, Bouwmeester H, Hamers FP, van der Linden AJ, Hellemons AJ, Graw J, Burbach JP (2004) Early developmental failure of substantia nigra dopamine neurons in mice lacking the homeodomain gene Pitx3. Development 131(5):1145–1155
Nunes I, Tovmasian LT, Silva RM, Burke RE, Goff SP (2003) Pitx3 is required for development of substantia nigra dopaminergic neurons. Proc Natl Acad Sci U S A 100(7):4245–4250
Hwang DY, Ardayfio P, Kang UJ, Semina EV, Kim KS (2003) Selective loss of dopaminergic neurons in the substantia nigra of Pitx3-deficient aphakia mice. Brain Res Mol Brain Res 114(2):123–131
van den Munckhof P, Luk KC, Ste-Marie L, Montgomery J, Blanchet PJ, Sadikot AF, Drouin J (2003) Pitx3 is required for motor activity and for survival of a subset of midbrain dopaminergic neurons. Development 130(11):2535–2542
Castillo SO, Baffi JS, Palkovits M, Goldstein DS, Kopin IJ, Witta J, Magnuson MA, Nikodem VM (1998) Dopamine biosynthesis is selectively abolished in substantia nigra/ventral tegmental area but not in hypothalamic neurons in mice with targeted disruption of the Nurr1 gene. Mol Cell Neurosci 11(1–2):36–46
Theofilopoulos S, Goggi J, Riaz SS, Jauniaux E, Stern GM, Bradford HF (2001) Parallel induction of the formation of dopamine and its metabolites with induction of tyrosine hydroxylase expression in foetal rat and human cerebral cortical cells by brain-derived neurotrophic factor and glial-cell derived neurotrophic factor. Brain Res Dev Brain Res 127(2):111–122
Moore MW, Klein RD, Farinas I, Sauer H, Armanini M, Phillips H, Reichardt LF, Ryan AM, Carver-Moore K, Rosenthal A (1996) Renal and neuronal abnormalities in mice lacking GDNF. Nature 382(6586):76–79
Pichel JG, Shen L, Sheng HZ, Granholm AC, Drago J, Grinberg A, Lee EJ, Huang SP, Saarma M, Hoffer BJ, Sariola H, Westphal H (1996) Defects in enteric innervation and kidney development in mice lacking GDNF. Nature 382(6586):73–76
Sanchez MP, Silos-Santiago I, Frisen J, He B, Lira SA, Barbacid M (1996) Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature 382(6586):70–73
Gates MA, Coupe VM, Torres EM, Fricker-Gates RA, Dunnett SB (2004) Spatially and temporally restricted chemoattractive and chemorepulsive cues direct the formation of the nigro-striatal circuit. Eur J Neurosci 19(4):831–844
Nakamura S, Ito Y, Shirasaki R, Murakami F (2000) Local directional cues control growth polarity of dopaminergic axons along the rostrocaudal axis. J Neurosci 20(11):4112–4119
Rosenblad C, Martinez-Serrano A, Bjorklund A (1998) Intrastriatal glial cell line-derived neurotrophic factor promotes sprouting of spared nigrostriatal dopaminergic afferents and induces recovery of function in a rat model of Parkinson’s disease. Neuroscience 82(1):129–137
Batchelor PE, Liberatore GT, Porritt MJ, Donnan GA, Howells DW (2000) Inhibition of brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor expression reduces dopaminergic sprouting in the injured striatum. Eur J Neurosci 12(10):3462–3468
Bourque MJ, Trudeau LE (2000) GDNF enhances the synaptic efficacy of dopaminergic neurons in culture. Eur J Neurosci 12(9):3172–3180
Martin D, Miller G, Cullen T, Fischer N, Dix D, Russell D (1996) Intranigral or intrastriatal injections of GDNF: effects on monoamine levels and behavior in rats. Eur J Pharmacol 317(2–3):247–256
Maswood N, Grondin R, Zhang Z, Stanford JA, Surgener SP, Gash DM, Gerhardt GA (2002) Effects of chronic intraputamenal infusion of glial cell line-derived neurotrophic factor (GDNF) in aged rhesus monkeys. Neurobiol Aging 23(5):881–889
Boger HA, Middaugh LD, Huang P, Zaman V, Smith AC, Hoffer BJ, Tomac AC, Granholm AC (2006) A partial GDNF depletion leads to earlier age-related deterioration of motor function and tyrosine hydroxylase expression in the substantia nigra. Exp Neurol 202(2):336–347
Horger BA, Nishimura MC, Armanini MP, Wang LC, Poulsen KT, Rosenblad C, Kirik D, Moffat B, Simmons L, Johnson E Jr, Milbrandt J, Rosenthal A, Bjorklund A, Vandlen RA, Hynes MA, Phillips HS (1998) Neurturin exerts potent actions on survival and function of midbrain dopaminergic neurons. J Neurosci 18(13):4929–4937
Akerud P, Alberch J, Eketjall S, Wagner J, Arenas E (1999) Differential effects of glial cell line-derived neurotrophic factor and neurturin on developing and adult substantia nigra dopaminergic neurons. J Neurochem 73(1):70–78
Tseng JL, Bruhn SL, Zurn AD, Aebischer P (1998) Neurturin protects dopaminergic neurons following medial forebrain bundle axotomy. Neuroreport 9(8):1817–1822
Hoane MR, Gulwadi AG, Morrison S, Hovanesian G, Lindner MD, Tao W (1999) Differential in vivo effects of neurturin and glial cell-line-derived neurotrophic factor. Exp Neurol 160(1):235–243
Oiwa Y, Yoshimura R, Nakai K, Itakura T (2002) Dopaminergic neuroprotection and regeneration by neurturin assessed by using behavioral, biochemical and histochemical measurements in a model of progressive Parkinson’s disease. Brain Res 947(2):271–283
Kordower JH, Herzog CD, Dass B, Bakay RA, Stansell J 3rd, Gasmi M, Bartus RT (2006) Delivery of neurturin by AAV2 (CERE-120)-mediated gene transfer provides structural and functional neuroprotection and neurorestoration in MPTP-treated monkeys. Ann Neurol 60(6):706–715
Herzog CD, Dass B, Holden JE, Stansell J 3rd, Gasmi M, Tuszynski MH, Bartus RT, Kordower JH (2007) Striatal delivery of CERE-120, an AAV2 vector encoding human neurturin, enhances activity of the dopaminergic nigrostriatal system in aged monkeys. Mov Disord 22(8):1124–1132
Marks WJ Jr, Ostrem JL, Verhagen L, Starr PA, Larson PS, Bakay RA, Taylor R, Cahn-Weiner DA, Stoessl AJ, Olanow CW, Bartus RT (2008) Safety and tolerability of intraputaminal delivery of CERE-120 (adeno-associated virus serotype 2-neurturin) to patients with idiopathic Parkinson’s disease: an open-label, phase I trial. Lancet Neurol 7(5):400–408
Marks WJ Jr, Bartus RT, Siffert J, Davis CS, Lozano A, Boulis N, Vitek J, Stacy M, Turner D, Verhagen L, Bakay R, Watts R, Guthrie B, Jankovic J, Simpson R, Tagliati M, Alterman R, Stern M, Baltuch G, Starr PA, Larson PS, Ostrem JL, Nutt J, Kieburtz K, Kordower JH, Olanow CW (2010) Gene delivery of AAV2-neurturin for Parkinson’s disease: a double-blind, randomised, controlled trial. Lancet Neurol 9(12):1164–1172
Bartus RT, Baumann TL, Siffert J, Herzog CD, Alterman R, Boulis N, Turner DA, Stacy M, Lang AE, Lozano AM, Olanow CW (2013) Safety/feasibility of targeting the substantia nigra with AAV2-neurturin in Parkinson patients. Neurology 80(18):1698–1701
Milbrandt J, de Sauvage FJ, Fahrner TJ, Baloh RH, Leitner ML, Tansey MG, Lampe PA, Heuckeroth RO, Kotzbauer PT, Simburger KS, Golden JP, Davies JA, Vejsada R, Kato AC, Hynes M, Sherman D, Nishimura M, Wang LC, Vandlen R, Moffat B, Klein RD, Poulsen K, Gray C, Garces A, Johnson EM Jr et al (1998) Persephin, a novel neurotrophic factor related to GDNF and neurturin. Neuron 20(2):245–253
Baloh RH, Tansey MG, Lampe PA, Fahrner TJ, Enomoto H, Simburger KS, Leitner ML, Araki T, Johnson EM Jr, Milbrandt J (1998) Artemin, a novel member of the GDNF ligand family, supports peripheral and central neurons and signals through the GFRalpha3-RET receptor complex. Neuron 21(6):1291–1302
Akerud P, Holm PC, Castelo-Branco G, Sousa K, Rodriguez FJ, Arenas E (2002) Persephin-overexpressing neural stem cells regulate the function of nigral dopaminergic neurons and prevent their degeneration in a model of Parkinson’s disease. Mol Cell Neurosci 21(2):205–222
Cass WA, Peters LE, Harned ME, Seroogy KB (2006) Protection by GDNF and other trophic factors against the dopamine-depleting effects of neurotoxic doses of methamphetamine. Ann N Y Acad Sci 1074:272–281
Zihlmann KB, Ducray AD, Schaller B, Huber AW, Krebs SH, Andres RH, Seiler RW, Meyer M, Widmer HR (2005) The GDNF family members neurturin, artemin and persephin promote the morphological differentiation of cultured ventral mesencephalic dopaminergic neurons. Brain Res Bull 68(1–2):42–53
Heuckeroth RO, Enomoto H, Grider JR, Golden JP, Hanke JA, Jackman A, Molliver DC, Bardgett ME, Snider WD, Johnson EM Jr, Milbrandt J (1999) Gene targeting reveals a critical role for neurturin in the development and maintenance of enteric, sensory, and parasympathetic neurons. Neuron 22(2):253–263
Tomac AC, Agulnick AD, Haughey N, Chang CF, Zhang Y, Backman C, Morales M, Mattson MP, Wang Y, Westphal H, Hoffer BJ (2002) Effects of cerebral ischemia in mice deficient in persephin. Proc Natl Acad Sci U S A 99(14):9521–9526
Honma Y, Araki T, Gianino S, Bruce A, Heuckeroth R, Johnson E, Milbrandt J (2002) Artemin is a vascular-derived neurotropic factor for developing sympathetic neurons. Neuron 35(2):267–282
Krieglstein K, Henheik P, Farkas L, Jaszai J, Galter D, Krohn K, Unsicker K (1998) Glial cell line-derived neurotrophic factor requires transforming growth factor-beta for exerting its full neurotrophic potential on peripheral and CNS neurons. J Neurosci 18(23):9822–9834
Schober A, Peterziel H, von Bartheld CS, Simon H, Krieglstein K, Unsicker K (2007) GDNF applied to the MPTP-lesioned nigrostriatal system requires TGF-beta for its neuroprotective action. Neurobiol Dis 25(2):378–391
Peterziel H, Unsicker K, Krieglstein K (2002) TGFbeta induces GDNF responsiveness in neurons by recruitment of GFRalpha1 to the plasma membrane. J Cell Biol 159(1):157–167
Unsicker K, Meier C, Krieglstein K, Sartor BM, Flanders KC (1996) Expression, localization, and function of transforming growth factor-beta s in embryonic chick spinal cord, hindbrain, and dorsal root ganglia. J Neurobiol 29(2):262–276
Flanders KC, Ludecke G, Engels S, Cissel DS, Roberts AB, Kondaiah P, Lafyatis R, Sporn MB, Unsicker K (1991) Localization and actions of transforming growth factor-beta s in the embryonic nervous system. Development 113(1):183–191
Krieglstein K, Unsicker K (1994) Transforming growth factor-beta promotes survival of midbrain dopaminergic neurons and protects them against N-methyl-4-phenylpyridinium ion toxicity. Neuroscience 63(4):1189–1196
Farkas LM, Dunker N, Roussa E, Unsicker K, Krieglstein K (2003) Transforming growth factor-beta(s) are essential for the development of midbrain dopaminergic neurons in vitro and in vivo. J Neurosci 23(12):5178–5186
Knoferle J, Ramljak S, Koch JC, Tonges L, Asif AR, Michel U, Wouters FS, Heermann S, Krieglstein K, Zerr I, Bahr M, Lingor P (2010) TGF-beta 1 enhances neurite outgrowth via regulation of proteasome function and EFABP. Neurobiol Dis 38(3):395–404
Roussa E, Wiehle M, Dunker N, Becker-Katins S, Oehlke O, Krieglstein K (2006) Transforming growth factor beta is required for differentiation of mouse mesencephalic progenitors into dopaminergic neurons in vitro and in vivo: ectopic induction in dorsal mesencephalon. Stem Cells 24(9):2120–2129
Li K, Xue B, Wang Y, Wang X, Wang H (2009) Ventral mesencephalon astrocytes are more efficient than those of other regions in inducing dopaminergic neurons through higher expression level of TGF-beta3. J Mol Neurosci 37(3):288–300
Castelo-Branco G, Sousa KM, Bryja V, Pinto L, Wagner J, Arenas E (2006) Ventral midbrain glia express region-specific transcription factors and regulate dopaminergic neurogenesis through Wnt-5a secretion. Mol Cell Neurosci 31(2):251–262
Roussa E, Oehlke O, Rahhal B, Heermann S, Heidrich S, Wiehle M, Krieglstein K (2008) Transforming growth factor beta cooperates with persephin for dopaminergic phenotype induction. Stem Cells 26(7):1683–1694
Sanford LP, Ormsby I, Gittenberger-de Groot AC, Sariola H, Friedman R, Boivin GP, Cardell EL, Doetschman T (1997) TGFbeta2 knockout mice have multiple developmental defects that are non-overlapping with other TGFbeta knockout phenotypes. Development 124(13):2659–2670
Kaartinen V, Voncken JW, Shuler C, Warburton D, Bu D, Heisterkamp N, Groffen J (1995) Abnormal lung development and cleft palate in mice lacking TGF-beta 3 indicates defects of epithelial-mesenchymal interaction. Nat Genet 11(4):415–421
Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D et al (1992) Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature 359(6397):693–699
Rahhal B, Heermann S, Ferdinand A, Rosenbusch J, Rickmann M, Krieglstein K (2009) In vivo requirement of TGF-beta/GDNF cooperativity in mouse development: focus on the neurotrophic hypothesis. Int J Dev Neurosci 27(1):97–102
Andrews ZB, Zhao H, Frugier T, Meguro R, Grattan DR, Koishi K, McLennan IS (2006) Transforming growth factor beta2 haploinsufficient mice develop age-related nigrostriatal dopamine deficits. Neurobiol Dis 21(3):568–575
Tapia-Gonzalez S, Giraldez-Perez RM, Cuartero MI, Casarejos MJ, Mena MA, Wang XF, Sanchez-Capelo A (2011) Dopamine and alpha-synuclein dysfunction in Smad3 null mice. Mol Neurodegener 6:72
Heermann S, Opazo F, Falkenburger B, Krieglstein K, Spittau B (2010) Aged Tgfbeta2/Gdnf double-heterozygous mice show no morphological and functional alterations in the nigrostriatal system. J Neural Transm 117(6):719–727
Zhang J, Pho V, Bonasera SJ, Holtzman J, Tang AT, Hellmuth J, Tang S, Janak PH, Tecott LH, Huang EJ (2007) Essential function of HIPK2 in TGFbeta-dependent survival of midbrain dopamine neurons. Nat Neurosci 10(1):77–86
Kawabata M, Imamura T, Miyazono K (1998) Signal transduction by bone morphogenetic proteins. Cytokine Growth Factor Rev 9(1):49–61
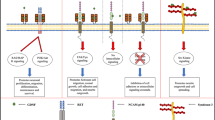
Hegarty SV, O’Keeffe GW, Sullivan AM (2013) BMP-Smad 1/5/8 signalling in the development of the nervous system. Prog Neurobiol 109C:28–41
Krieglstein K, Suter-Crazzolara C, Hotten G, Pohl J, Unsicker K (1995) Trophic and protective effects of growth/differentiation factor 5, a member of the transforming growth factor-beta superfamily, on midbrain dopaminergic neurons. J Neurosci Res 42(5):724–732
O’Keeffe GW, Hanke M, Pohl J, Sullivan AM (2004) Expression of growth differentiation factor-5 in the developing and adult rat brain. Brain Res Dev Brain Res 151(1–2):199–202
Storm EE, Huynh TV, Copeland NG, Jenkins NA, Kingsley DM, Lee SJ (1994) Limb alterations in brachypodism mice due to mutations in a new member of the TGF beta-superfamily. Nature 368(6472):639–643
O’Keeffe GW, Dockery P, Sullivan AM (2004) Effects of growth/differentiation factor 5 on the survival and morphology of embryonic rat midbrain dopaminergic neurones in vitro. J Neurocytol 33(5):479–488
Wood TK, McDermott KW, Sullivan AM (2005) Differential effects of growth/differentiation factor 5 and glial cell line-derived neurotrophic factor on dopaminergic neurons and astroglia in cultures of embryonic rat midbrain. J Neurosci Res 80(6):759–766
Clayton KB, Sullivan AM (2007) Differential effects of GDF5 on the medial and lateral rat ventral mesencephalon. Neurosci Lett 427(3):132–137
O’Sullivan DB, Harrison PT, Sullivan AM (2010) Effects of GDF5 overexpression on embryonic rat dopaminergic neurones in vitro and in vivo. J Neural Transm 117(5):559–572
Lingor P, Unsicker K, Krieglstein K (1999) Midbrain dopaminergic neurons are protected from radical induced damage by GDF-5 application. Short communication. J Neural Transm 106(2):139–144
O’Keeffe GW, Dockery P, Sullivan AM (2004) Effects of growth/differentiation factor 5 on the survival and morphology of embryonic rat midbrain dopaminergic neurones in vitro. J Neurocytol 33(5):479–488
Hegarty SV, Sullivan AM, O’Keeffe GW (2013) BMP2 and GDF5 induce neuronal differentiation through a Smad dependant pathway in a model of human midbrain dopaminergic neurons. Mol Cell Neurosci 56C:263–271
Nishitoh H, Ichijo H, Kimura M, Matsumoto T, Makishima F, Yamaguchi A, Yamashita H, Enomoto S, Miyazono K (1996) Identification of type I and type II serine/threonine kinase receptors for growth/differentiation factor-5. J Biol Chem 271(35):21345–21352
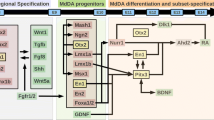
Abeliovich A, Hammond R (2007) Midbrain dopamine neuron differentiation: factors and fates. Dev Biol 304(2):447–454
Sullivan AM, Opacka-Juffry J, Hotten G, Pohl J, Blunt SB (1997) Growth/differentiation factor 5 protects nigrostriatal dopaminergic neurones in a rat model of Parkinson’s disease. Neurosci Lett 233(2–3):73–76
Sullivan AM, Opacka-Juffry J, Pohl J, Blunt SB (1999) Neuroprotective effects of growth/differentiation factor 5 depend on the site of administration. Brain Res 818(1):176–179
Hurley FM, Costello DJ, Sullivan AM (2004) Neuroprotective effects of delayed administration of growth/differentiation factor-5 in the partial lesion model of Parkinson’s disease. Exp Neurol 185(2):281–289
Costello DJ, O’Keeffe GW, Hurley FM, Sullivan AM (2012) Transplantation of novel human GDF5-expressing CHO cells is neuroprotective in models of Parkinson’s disease. J Cell Mol Med 16(10):2451–2460
Sullivan AM, Opacka-Juffry J, Blunt SB (1998) Long-term protection of the rat nigrostriatal dopaminergic system by glial cell line- derived neurotrophic factor against 6hydroxydopamine in vivo. Eur J Neurosci 10(1):57–63
Chou J, Harvey BK, Ebendal T, Hoffer B, Wang Y (2008) Nigrostriatal alterations in bone morphogenetic protein receptor II dominant negative mice. Acta Neurochir Suppl 101:93–98
Hegarty SV, Collins LM, Gavin AM, Roche SL, Wyatt SL, Sullivan AM, O’Keeffe GW (2014) Canonical BMP-Smad signalling promotes neurite growth in midbrain dopaminergic neurons. NeuroMolecular Med (in revision)
Barnett MW, Fisher CE, Perona-Wright G, Davies JA (2002) Signalling by glial cell line-derived neurotrophic factor (GDNF) requires heparan sulphate glycosaminoglycan. J Cell Sci 115(Pt 23):4495–4503
Iwase T, Jung CG, Bae H, Zhang M, Soliven B (2005) Glial cell line-derived neurotrophic factor-induced signaling in Schwann cells. J Neurochem 94(6):1488–1499
Strelau J, Sullivan A, Bottner M, Lingor P, Falkenstein E, Suter-Crazzolara C, Galter D, Jaszai J, Krieglstein K, Unsicker K (2000) Growth/differentiation factor-15/macrophage inhibitory cytokine-1 is a novel trophic factor for midbrain dopaminergic neurons in vivo. J Neurosci 20(23):8597–8603
Brederlau A, Faigle R, Kaplan P, Odin P, Funa K (2002) Bone morphogenetic proteins but not growth differentiation factors induce dopaminergic differentiation in mesencephalic precursors. Mol Cell Neurosci 21(3):367–378
Jordan J, Bottner M, Schluesener HJ, Unsicker K, Krieglstein K (1997) Bone morphogenetic proteins: neurotrophic roles for midbrain dopaminergic neurons and implications of astroglial cells. Eur J Neurosci 9(8):1699–1709
Soderstrom S, Ebendal T (1999) Localized expression of BMP and GDF mRNA in the rodent brain. J Neurosci Res 56(5):482–492
Chen HL, Lein PJ, Wang JY, Gash D, Hoffer BJ, Chiang YH (2003) Expression of bone morphogenetic proteins in the brain during normal aging and in 6-hydroxydopamine-lesioned animals. Brain Res 994(1):81–90
Reiriz J, Espejo M, Ventura F, Ambrosio S, Alberch J (1999) Bone morphogenetic protein-2 promotes dissociated effects on the number and differentiation of cultured ventral mesencephalic dopaminergic neurons. J Neurobiol 38(2):161–170
Espejo M, Cutillas B, Ventura F, Ambrosio S (1999) Exposure of foetal mesencephalic cells to bone morphogenetic protein-2 enhances the survival of dopaminergic neurones in rat striatal grafts. Neurosci Lett 275(1):13–16
Lee JY, Koh HC, Chang MY, Park CH, Lee YS, Lee SH (2003) Erythropoietin and bone morphogenetic protein 7 mediate ascorbate-induced dopaminergic differentiation from embryonic mesencephalic precursors. Neuroreport 14(10):1401–1404
Harvey BK, Mark A, Chou J, Chen GJ, Hoffer BJ, Wang Y (2004) Neurotrophic effects of bone morphogenetic protein-7 in a rat model of Parkinson’s disease. Brain Res 1022(1–2):88–95
Chou J, Luo Y, Kuo CC, Powers K, Shen H, Harvey BK, Hoffer BJ, Wang Y (2008) Bone morphogenetic protein-7 reduces toxicity induced by high doses of methamphetamine in rodents. Neuroscience 151(1):92–103
Andersson E, Tryggvason U, Deng Q, Friling S, Alekseenko Z, Robert B, Perlmann T, Ericson J (2006) Identification of intrinsic determinants of midbrain dopamine neurons. Cell 124(2):393–405
Alavian KN, Scholz C, Simon HH (2008) Transcriptional regulation of mesencephalic dopaminergic neurons: the full circle of life and death. Mov Disord 23(3):319–328
Maeda R, Kobayashi A, Sekine R, Lin JJ, Kung H, Maeno M (1997) Xmsx-1 modifies mesodermal tissue pattern along dorsoventral axis in Xenopus laevis embryo. Development 124(13):2553–2560
Tribulo C, Aybar MJ, Nguyen VH, Mullins MC, Mayor R (2003) Regulation of Msx genes by a Bmp gradient is essential for neural crest specification. Development 130(26):6441–6452
Chizhikov VV, Millen KJ (2004) Control of roof plate formation by Lmx1a in the developing spinal cord (Lmx1a + BMPs). Development 131(11):2693–2705
Liu Y, Helms AW, Johnson JE (2004) Distinct activities of Msx1 and Msx3 in dorsal neural tube development. Development 131(5):1017–1028
Roussa E, Krieglstein K (2004) Induction and specification of midbrain dopaminergic cells: focus on SHH, FGF8, and TGF-beta. Cell Tissue Res 318(1):23–33
Joksimovic M, Yun BA, Kittappa R, Anderegg AM, Chang WW, Taketo MM, McKay RD, Awatramani RB (2009) Wnt antagonism of Shh facilitates midbrain floor plate neurogenesis. Nat Neurosci 12(2):125–131
LaBonne C, Bronner-Fraser M (1998) Neural crest induction in Xenopus: evidence for a two-signal model. Development 125(13):2403–2414
Monsoro-Burq AH, Wang E, Harland R (2005) Msx1 and Pax3 cooperate to mediate FGF8 and WNT signals during Xenopus neural crest induction. Dev Cell 8(2):167–178
Liu A, Niswander LA (2005) Bone morphogenetic protein signalling and vertebrate nervous system development. Nat Rev Neurosci 6(12):945–954
Eivers E, Fuentealba LC, De Robertis EM (2008) Integrating positional information at the level of Smad1/5/8. Curr Opin Genet Dev 18(4):304–310
Fuentealba LC, Eivers E, Ikeda A, Hurtado C, Kuroda H, Pera EM, De Robertis EM (2007) Integrating patterning signals: Wnt/GSK3 regulates the duration of the BMP/Smad1 signal. Cell 131(5):980–993
Bonilla S, Hall AC, Pinto L, Attardo A, Gotz M, Huttner WB, Arenas E (2008) Identification of midbrain floor plate radial glia-like cells as dopaminergic progenitors. Glia 56(8):809–820
Hebsgaard JB, Nelander J, Sabelstrom H, Jonsson ME, Stott S, Parmar M (2009) Dopamine neuron precursors within the developing human mesencephalon show radial glial characteristics. Glia 57(15):1648–1658
Altmann CR, Brivanlou AH (2001) Neural patterning in the vertebrate embryo. Int Rev Cytol 203:447–482
Ulloa F, Briscoe J (2007) Morphogens and the control of cell proliferation and patterning in the spinal cord. Cell Cycle 6(21):2640–2649
Jessell TM (2000) Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat Rev Genet 1(1):20–29
Placzek M, Briscoe J (2005) The floor plate: multiple cells, multiple signals. Nat Rev Neurosci 6(3):230–240
Fuccillo M, Joyner AL, Fishell G (2006) Morphogen to mitogen: the multiple roles of hedgehog signalling in vertebrate neural development. Nat Rev Neurosci 7(10):772–783
Echelard Y, Vassileva G, McMahon AP (1994) Cis-acting regulatory sequences governing Wnt-1 expression in the developing mouse CNS. Development 120(8):2213–2224
Castelo-Branco G, Wagner J, Rodriguez FJ, Kele J, Sousa K, Rawal N, Pasolli HA, Fuchs E, Kitajewski J, Arenas E (2003) Differential regulation of midbrain dopaminergic neuron development by Wnt-1, Wnt-3a, and Wnt-5a. Proc Natl Acad Sci U S A 100(22):12747–12752
Zervas M, Millet S, Ahn S, Joyner AL (2004) Cell behaviors and genetic lineages of the mesencephalon and rhombomere 1. Neuron 43(3):345–357
Prakash N, Brodski C, Naserke T, Puelles E, Gogoi R, Hall A, Panhuysen M, Echevarria D, Sussel L, Weisenhorn DM, Martinez S, Arenas E, Simeone A, Wurst W (2006) A Wnt1-regulated genetic network controls the identity and fate of midbrain-dopaminergic progenitors in vivo. Development 133(1):89–98
Chesnutt C, Burrus LW, Brown AM, Niswander L (2004) Coordinate regulation of neural tube patterning and proliferation by TGFbeta and WNT activity. Dev Biol 274(2):334–347
Wine-Lee L, Ahn KJ, Richardson RD, Mishina Y, Lyons KM, Crenshaw EB 3rd (2004) Signaling through BMP type 1 receptors is required for development of interneuron cell types in the dorsal spinal cord. Development 131(21):5393–5403
Failli V, Bachy I, Retaux S (2002) Expression of the LIM-homeodomain gene Lmx1a (dreher) during development of the mouse nervous system. Mech Dev 118(1–2):225–228
Chizhikov VV, Lindgren AG, Mishima Y, Roberts RW, Aldinger KA, Miesegaes GR, Currle DS, Monuki ES, Millen KJ (2010) Lmx1a regulates fates and location of cells originating from the cerebellar rhombic lip and telencephalic cortical hem. Proc Natl Acad Sci U S A 107(23):10725–10730
Griesel G, Krug C, Yurlova L, Diaconu M, Mansouri A (2011) Generation of knockout mice expressing a GFP-reporter under the control of the Lmx1a locus. Gene Expr Patterns 11(5–6):345–348
Alder J, Lee KJ, Jessell TM, Hatten ME (1999) Generation of cerebellar granule neurons in vivo by transplantation of BMP-treated neural progenitor cells. Nat Neurosci 2(6):535–540
Qin L, Wine-Lee L, Ahn KJ, Crenshaw EB 3rd (2006) Genetic analyses demonstrate that bone morphogenetic protein signaling is required for embryonic cerebellar development. J Neurosci 26(7):1896–1905
Dale K, Sattar N, Heemskerk J, Clarke JD, Placzek M, Dodd J (1999) Differential patterning of ventral midline cells by axial mesoderm is regulated by BMP7 and chordin. Development 126(2):397–408
Yung SY, Gokhan S, Jurcsak J, Molero AE, Abrajano JJ, Mehler MF (2002) Differential modulation of BMP signaling promotes the elaboration of cerebral cortical GABAergic neurons or oligodendrocytes from a common sonic hedgehog-responsive ventral forebrain progenitor species. Proc Natl Acad Sci U S A 99(25):16273–16278
Wallace JA, Lauder JM (1983) Development of the serotonergic system in the rat embryo: an immunocytochemical study. Brain Res Bull 10(4):459–479
Wilson SI, Graziano E, Harland R, Jessell TM, Edlund T (2000) An early requirement for FGF signalling in the acquisition of neural cell fate in the chick embryo. Curr Biol 10(8):421–429
Streit A, Berliner AJ, Papanayotou C, Sirulnik A, Stern CD (2000) Initiation of neural induction by FGF signalling before gastrulation. Nature 406(6791):74–78
Pera EM, Ikeda A, Eivers E, De Robertis EM (2003) Integration of IGF, FGF, and anti-BMP signals via Smad1 phosphorylation in neural induction. Genes Dev 17(24):3023–3028
Acknowledgments
Work in the authors’ laboratories is supported by grants from the Irish Research Council (R13702; SVH/AS/G’OK), the Health Research Board of Ireland (HRA/2009/127; GO’K/AS) and Science Foundation Ireland (10/RFP/NES2786; GO’K).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Aideen Sullivan and Gerard O’Keeffe contributed equally to this work.
Rights and permissions
About this article
Cite this article
Hegarty, S.V., Sullivan, A.M. & O’Keeffe, G.W. Roles for the TGFβ Superfamily in the Development and Survival of Midbrain Dopaminergic Neurons. Mol Neurobiol 50, 559–573 (2014). https://doi.org/10.1007/s12035-014-8639-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-014-8639-3