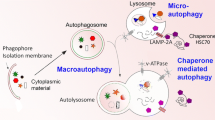
Abstract
Regular protein synthesis is a needful and complex task for a healthy cell. Improper folding leads to the deposition of misfolded proteins in cells. Autophagy and ubiquitin–proteasome system (UPS) are the conserved intracellular degradation processes of eukaryotic cells. How exactly these two pathways cross talk to each other is unclear. We do not know how the impairment of autophagy or UPS leads to the disturbance in cellular homeostasis and contribute into cellular aging and neurodegeneration. Here in this review, we will focus on the functional interconnections of autophagy and UPS, and why their loss of function results in abnormal aggregation of misfolded proteotoxic species in cells. Finally, we enumerate and discuss the crucial inducers of autophagy pathways and elaborate their intersection steps, which have been considered to be advantageous in aging linked with the abnormal protein aggregation. The final goal of this review is to improve our current understanding about multifaceted properties and interactions of autophagy and UPS, which may provide new insights to identify novel therapeutic strategies for aging and neurodegenerative diseases.




Similar content being viewed by others
References
Goldberg AL (2003) Protein degradation and protection against misfolded or damaged proteins. Nature 426(6968):895–899. doi:10.1038/nature02263 nature02263
Mizushima N (2007) Autophagy: process and function. Genes Dev 21(22):2861–2873. doi:10.1101/gad.1599207
Pechmann S, Willmund F, Frydman J (2013) The ribosome as a hub for protein quality control. Mol Cell 49(3):411–421. doi:10.1016/j.molcel.2013.01.020
Liu B, Han Y, Qian SB (2013) Cotranslational response to proteotoxic stress by elongation pausing of ribosomes. Mol Cell 49(3):453–463. doi:10.1016/j.molcel.2012.12.001
Dou F, Netzer WJ, Tanemura K, Li F, Hartl FU, Takashima A, Gouras GK, Greengard P, Xu H (2003) Chaperones increase association of tau protein with microtubules. Proc Natl Acad Sci U S A 100(2):721–726. doi:10.1073/pnas.242720499 242720499
Mishra A, Dikshit P, Purkayastha S, Sharma J, Nukina N, Jana NR (2008) E6-AP promotes misfolded polyglutamine proteins for proteasomal degradation and suppresses polyglutamine protein aggregation and toxicity. J Biol Chem 283(12):7648–7656. doi:10.1074/jbc.M706620200
Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H, Overvatn A, Bjorkoy G, Johansen T (2007) p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem 282(33):24131–24145. doi:10.1074/jbc.M702824200
Chhangani D, Mishra A (2013) Mahogunin ring finger-1 (MGRN1) suppresses chaperone-associated misfolded protein aggregation and toxicity. Sci Rep 3:1972. doi:10.1038/srep01972
Yamanaka T, Nukina N (2010) Transcription factor sequestration by polyglutamine proteins. Methods Mol Biol 648:215–229. doi:10.1007/978-1-60761-756-3_14
Preissler S, Deuerling E (2012) Ribosome-associated chaperones as key players in proteostasis. Trends Biochem Sci 37(7):274–283. doi:10.1016/j.tibs.2012.03.002
Duttler S, Pechmann S, Frydman J (2013) Principles of cotranslational ubiquitination and quality control at the ribosome. Mol Cell 50(3):379–393. doi:10.1016/j.molcel.2013.03.010
Nedelsky NB, Todd PK, Taylor JP (2008) Autophagy and the ubiquitin–proteasome system: collaborators in neuroprotection. Biochim Biophys Acta 1782(12):691–699. doi:10.1016/j.bbadis.2008.10.002
Pandey UB, Batlevi Y, Baehrecke EH, Taylor JP (2007) HDAC6 at the intersection of autophagy, the ubiquitin–proteasome system and neurodegeneration. Autophagy 3(6):643–645
Aguzzi A, O’Connor T (2010) Protein aggregation diseases: pathogenicity and therapeutic perspectives. Nat Rev Drug Discov 9(3):237–248. doi:10.1038/nrd3050
Zheng Q, Li J, Wang X (2009) Interplay between the ubiquitin–proteasome system and autophagy in proteinopathies. Int J Physiol Pathophysiol Pharmacol 1(2):127–142
Lilienbaum A (2013) Relationship between the proteasomal system and autophagy. Int J Biochem Mol Biol 4(1):1–26
Wang XJ, Yu J, Wong SH, Cheng AS, Chan FK, Ng SS, Cho CH, Sung JJ, Wu WK (2013) A novel crosstalk between two major protein degradation systems: regulation of proteasomal activity by autophagy. Autophagy 9 (10). doi: 10.4161/auto.25573
Ihara Y, Morishima-Kawashima M, Nixon R (2012) The ubiquitin–proteasome system and the autophagic–lysosomal system in Alzheimer disease. Cold Spring Harb Perspect Med 2 (8). doi:10.1101/cshperspect.a006361
Matsuda N, Tanaka K (2010) Does impairment of the ubiquitin–proteasome system or the autophagy-lysosome pathway predispose individuals to neurodegenerative disorders such as Parkinson’s disease? J Alzheimers Dis 19(1):1–9. doi:10.3233/JAD-2010-1231
Taylor JP, Hardy J, Fischbeck KH (2002) Toxic proteins in neurodegenerative disease. Science 296(5575):1991–1995. doi:10.1126/science.1067122
Lamark T, Johansen T (2012) Aggrephagy: selective disposal of protein aggregates by macroautophagy. Int J Cell Biol 2012:736905. doi:10.1155/2012/736905
Bove J, Zhou C, Jackson-Lewis V, Taylor J, Chu Y, Rideout HJ, Wu DC, Kordower JH, Petrucelli L, Przedborski S (2006) Proteasome inhibition and Parkinson’s disease modeling. Ann Neurol 60(2):260–264. doi:10.1002/ana.20937
Chung KK, Dawson VL, Dawson TM (2001) The role of the ubiquitin–proteasomal pathway in Parkinson’s disease and other neurodegenerative disorders. Trends Neurosci 24(11 Suppl):S7–S14
Webb JL, Ravikumar B, Atkins J, Skepper JN, Rubinsztein DC (2003) Alpha-Synuclein is degraded by both autophagy and the proteasome. J Biol Chem 278(27):25009–25013. doi:10.1074/jbc.M300227200
Engelender S (2012) alpha-Synuclein fate: proteasome or autophagy? Autophagy 8(3):418–420. doi:10.4161/auto.19085
Kiffin R, Christian C, Knecht E, Cuervo AM (2004) Activation of chaperone-mediated autophagy during oxidative stress. Mol Biol Cell 15(11):4829–4840. doi:10.1091/mbc.E04-06-0477
Ding WX, Ni HM, Gao W, Yoshimori T, Stolz DB, Ron D, Yin XM (2007) Linking of autophagy to ubiquitin–proteasome system is important for the regulation of endoplasmic reticulum stress and cell viability. Am J Pathol 171(2):513–524. doi:10.2353/ajpath.2007.070188
Bernales S, McDonald KL, Walter P (2006) Autophagy counterbalances endoplasmic reticulum expansion during the unfolded protein response. PLoS Biol 4(12):e423. doi:10.1371/journal.pbio.0040423
Schubert U, Anton LC, Gibbs J, Norbury CC, Yewdell JW, Bennink JR (2000) Rapid degradation of a large fraction of newly synthesized proteins by proteasomes. Nature 404(6779):770–774. doi:10.1038/35008096
Chhangani D, Joshi AP, Mishra A (2012) E3 ubiquitin ligases in protein quality control mechanism. Mol Neurobiol 45(3):571–585. doi:10.1007/s12035-012-8273-x
Rabinowitz JD, White E (2010) Autophagy and metabolism. Science 330(6009):1344–1348. doi:10.1126/science.1193497
Kroemer G, Marino G, Levine B (2010) Autophagy and the integrated stress response. Mol Cell 40(2):280–293. doi:10.1016/j.molcel.2010.09.023
Yamamoto A, Simonsen A (2011) The elimination of accumulated and aggregated proteins: a role for aggrephagy in neurodegeneration. Neurobiol Dis 43(1):17–28. doi:10.1016/j.nbd.2010.08.015
Johnston JA, Ward CL, Kopito RR (1998) Aggresomes: a cellular response to misfolded proteins. J Cell Biol 143(7):1883–1898
Mitraki A (2010) Protein aggregation from inclusion bodies to amyloid and biomaterials. Adv Protein Chem Struct Biol 79:89–125. doi:10.1016/S1876-1623(10)79003-9
Snyder H, Mensah K, Theisler C, Lee J, Matouschek A, Wolozin B (2003) Aggregated and monomeric alpha-synuclein bind to the S6′ proteasomal protein and inhibit proteasomal function. J Biol Chem 278(14):11753–11759. doi:10.1074/jbc.M208641200
Bence NF, Sampat RM, Kopito RR (2001) Impairment of the ubiquitin–proteasome system by protein aggregation. Science 292(5521):1552–1555. doi:10.1126/science.292.5521.1552
Andre R, Tabrizi SJ (2012) Misfolded PrP and a novel mechanism of proteasome inhibition. Prion 6(1):32–36. doi:10.4161/pri.6.1.18272
Low P, Varga A, Pircs K, Nagy P, Szatmari Z, Sass M, Juhasz G (2013) Impaired proteasomal degradation enhances autophagy via hypoxia signaling in Drosophila. BMC Cell Biol 14(1):29. doi:10.1186/1471-2121-14-29
Kraft C, Peter M, Hofmann K (2010) Selective autophagy: ubiquitin-mediated recognition and beyond. Nat Cell Biol 12(9):836–841. doi:10.1038/ncb0910-836
McNaught KS, Olanow CW, Halliwell B, Isacson O, Jenner P (2001) Failure of the ubiquitin–proteasome system in Parkinson’s disease. Nat Rev Neurosci 2(8):589–594. doi:10.1038/35086067
Mizushima N, Levine B, Cuervo AM, Klionsky DJ (2008) Autophagy fights disease through cellular self-digestion. Nature 451(7182):1069–1075. doi:10.1038/nature06639
Nassif M, Hetz C (2012) Autophagy impairment: a crossroad between neurodegeneration and tauopathies. BMC Biol 10:78. doi:10.1186/1741-7007-10-78
Levine B, Kroemer G (2008) Autophagy in the pathogenesis of disease. Cell 132(1):27–42. doi:10.1016/j.cell.2007.12.018
Kirkin V, McEwan DG, Novak I, Dikic I (2009) A role for ubiquitin in selective autophagy. Mol Cell 34(3):259–269. doi:10.1016/j.molcel.2009.04.026
Chhangani D, Mishra A (2013) Protein quality control system in neurodegeneration: a healing company hard to beat but failure is fatal. Mol Neurobiol 48(1):141–156. doi:10.1007/s12035-013-8411-0
Chhangani D, Jana NR, Mishra A (2013) Misfolded proteins recognition strategies of E3 ubiquitin ligases and neurodegenerative diseases. Mol Neurobiol 47(1):302–312. doi:10.1007/s12035-012-8351-0
Narendra D, Walker JE, Youle R (2012) Mitochondrial quality control mediated by PINK1 and Parkin: links to parkinsonism. Cold Spring Harb Perspect Biol 4 (11). doi:10.1101/cshperspect.a011338
Wenzel DM, Lissounov A, Brzovic PS, Klevit RE (2011) UBCH7 reactivity profile reveals parkin and HHARI to be RING/HECT hybrids. Nature 474(7349):105–108. doi:10.1038/nature09966
Youle RJ, Narendra DP (2011) Mechanisms of mitophagy. Nat Rev Mol Cell Biol 12(1):9–14. doi:10.1038/nrm3028
Narendra D, Tanaka A, Suen DF, Youle RJ (2008) Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol 183(5):795–803. doi:10.1083/jcb.200809125
Sarraf SA, Raman M, Guarani-Pereira V, Sowa ME, Huttlin EL, Gygi SP, Harper JW (2013) Landscape of the PARKIN-dependent ubiquitylome in response to mitochondrial depolarization. Nature 496(7445):372–376. doi:10.1038/nature12043
Olzmann JA, Chin LS (2008) Parkin-mediated K63-linked polyubiquitination: a signal for targeting misfolded proteins to the aggresome-autophagy pathway. Autophagy 4(1):85–87
Chin LS, Olzmann JA, Li L (2010) Parkin-mediated ubiquitin signalling in aggresome formation and autophagy. Biochem Soc Trans 38(Pt 1):144–149. doi:10.1042/BST0380144
Herman AM, Moussa CE (2011) The ubiquitin ligase parkin modulates the execution of autophagy. Autophagy 7(8):919–921
Twig G, Hyde B, Shirihai OS (2008) Mitochondrial fusion, fission and autophagy as a quality control axis: the bioenergetic view. Biochim Biophys Acta 1777(9):1092–1097. doi:10.1016/j.bbabio.2008.05.001
Jin S (2006) Autophagy, mitochondrial quality control, and oncogenesis. Autophagy 2(2):80–84
Kundu M, Thompson CB (2005) Macroautophagy versus mitochondrial autophagy: a question of fate? Cell Death Differ 12(Suppl 2):1484–1489. doi:10.1038/sj.cdd.4401780
Lemasters JJ (2005) Selective mitochondrial autophagy, or mitophagy, as a targeted defense against oxidative stress, mitochondrial dysfunction, and aging. Rejuvenation Res 8(1):3–5. doi:10.1089/rej.2005.8.3
Lee J, Giordano S, Zhang J (2012) Autophagy, mitochondria and oxidative stress: cross-talk and redox signalling. Biochem J 441(2):523–540. doi:10.1042/BJ20111451
Tang F, Wang B, Li N, Wu Y, Jia J, Suo T, Chen Q, Liu YJ, Tang J (2011) RNF185, a novel mitochondrial ubiquitin E3 ligase, regulates autophagy through interaction with BNIP1. PLoS One 6(9):e24367. doi:10.1371/journal.pone.0024367
Cleveland DW, Rothstein JD (2001) From Charcot to Lou Gehrig: deciphering selective motor neuron death in ALS. Nat Rev Neurosci 2(11):806–819. doi:10.1038/35097565
Mishra A, Maheshwari M, Chhangani D, Fujimori-Tonou N, Endo F, Joshi AP, Jana NR, Yamanaka K (2013) E6-AP association promotes SOD1 aggresomes degradation and suppresses toxicity. Neurobiol Aging 34(4):1310 e1311–1323. doi:10.1016/j.neurobiolaging.2012.08.016
Mishra A, Godavarthi SK, Maheshwari M, Goswami A, Jana NR (2009) The ubiquitin ligase E6-AP is induced and recruited to aggresomes in response to proteasome inhibition and may be involved in the ubiquitination of Hsp70-bound misfolded proteins. J Biol Chem 284(16):10537–10545. doi:10.1074/jbc.M806804200
Morito D, Hirao K, Oda Y, Hosokawa N, Tokunaga F, Cyr DM, Tanaka K, Iwai K, Nagata K (2008) Gp78 cooperates with RMA1 in endoplasmic reticulum-associated degradation of CFTRDeltaF508. Mol Biol Cell 19(4):1328–1336. doi:10.1091/mbc.E07-06-0601
Ying Z, Wang H, Fan H, Zhu X, Zhou J, Fei E, Wang G (2009) Gp78, an ER associated E3, promotes SOD1 and ataxin-3 degradation. Hum Mol Genet 18(22):4268–4281. doi:10.1093/hmg/ddp380
Tsai YC, Mendoza A, Mariano JM, Zhou M, Kostova Z, Chen B, Veenstra T, Hewitt SM, Helman LJ, Khanna C, Weissman AM (2007) The ubiquitin ligase gp78 promotes sarcoma metastasis by targeting KAI1 for degradation. Nat Med 13(12):1504–1509. doi:10.1038/nm1686
Fu M, St-Pierre P, Shankar J, Wang PT, Joshi B, Nabi IR (2013) Regulation of mitophagy by the Gp78 E3 ubiquitin ligase. Mol Biol Cell 24(8):1153–1162. doi:10.1091/mbc.E12-08-0607
Wong E, Cuervo AM (2010) Autophagy gone awry in neurodegenerative diseases. Nat Neurosci 13(7):805–811. doi:10.1038/nn.2575
Kroemer G, Levine B (2008) Autophagic cell death: the story of a misnomer. Nat Rev Mol Cell Biol 9(12):1004–1010. doi:10.1038/nrm2529
Buchan JR, Kolaitis RM, Taylor JP, Parker R (2013) Eukaryotic stress granules are cleared by autophagy and Cdc48/VCP function. Cell 153(7):1461–1474. doi:10.1016/j.cell.2013.05.037
Qiang L, Wu C, Ming M, Viollet B, He YY (2013) Autophagy controls p38 activation to promote cell survival under genotoxic stress. J Biol Chem 288(3):1603–1611. doi:10.1074/jbc.M112.415224
Hansen M, Chandra A, Mitic LL, Onken B, Driscoll M, Kenyon C (2008) A role for autophagy in the extension of lifespan by dietary restriction in C. elegans. PLoS Genet 4(2):e24
Williams A, Sarkar S, Cuddon P, Ttofi EK, Saiki S, Siddiqi FH, Jahreiss L, Fleming A, Pask D, Goldsmith P, O’Kane CJ, Floto RA, Rubinsztein DC (2008) Novel targets for Huntington’s disease in an mTOR-independent autophagy pathway. Nat Chem Biol 4(5):295–305. doi:10.1038/nchembio.79
Kabuta T, Suzuki Y, Wada K (2006) Degradation of amyotrophic lateral sclerosis-linked mutant Cu, Zn-superoxide dismutase proteins by macroautophagy and the proteasome. J Biol Chem 281(41):30524–30533. doi:10.1074/jbc.M603337200
Hamano T, Gendron TF, Causevic E, Yen SH, Lin WL, Isidoro C, Deture M, Ko LW (2008) Autophagic-lysosomal perturbation enhances tau aggregation in transfectants with induced wild-type tau expression. Eur J Neurosci 27(5):1119–1130. doi:10.1111/j.1460-9568.2008.06084.x
Jimenez-Sanchez M, Thomson F, Zavodszky E, Rubinsztein DC (2012) Autophagy and polyglutamine diseases. Prog Neurobiol 97(2):67–82. doi:10.1016/j.pneurobio.2011.08.013
Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, Mizushima N (2006) Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 441(7095):885–889. doi:10.1038/nature04724
Schaeffer V, Lavenir I, Ozcelik S, Tolnay M, Winkler DT, Goedert M (2012) Stimulation of autophagy reduces neurodegeneration in a mouse model of human tauopathy. Brain 135(Pt 7):2169–2177. doi:10.1093/brain/aws143
Martinez-Vicente M, Cuervo AM (2007) Autophagy and neurodegeneration: when the cleaning crew goes on strike. Lancet Neurol 6(4):352–361. doi:10.1016/S1474-4422(07)70076-5
Bergamini E (2006) Autophagy: a cell repair mechanism that retards ageing and age-associated diseases and can be intensified pharmacologically. Mol Aspects Med 27(5–6):403–410. doi:10.1016/j.mam.2006.08.001
Cuervo AM (2008) Autophagy and aging: keeping that old broom working. Trends Genet 24(12):604–612. doi:10.1016/j.tig.2008.10.002
Vellai T (2009) Autophagy genes and ageing. Cell Death Differ 16(1):94–102. doi:10.1038/cdd.2008.126
Melendez A, Talloczy Z, Seaman M, Eskelinen EL, Hall DH, Levine B (2003) Autophagy genes are essential for dauer development and life-span extension in C. elegans. Science 301(5638):1387–1391. doi:10.1126/science.1087782
Liu Y, Shi S, Gu Z, Du Y, Liu M, Yan S, Gao J, Li J, Shao Y, Zhong W, Chen X, Li C (2012) Impaired autophagic function in rat islets with aging. Age (Dordr). doi:10.1007/s11357-012-9456-0
Hekimi S, Guarente L (2003) Genetics and the specificity of the aging process. Science 299(5611):1351–1354. doi:10.1126/science.1082358
Kirkwood TB (2008) A systematic look at an old problem. Nature 451(7179):644–647. doi:10.1038/451644a
Bernardes de Jesus B, Vera E, Schneeberger K, Tejera AM, Ayuso E, Bosch F, Blasco MA (2012) Telomerase gene therapy in adult and old mice delays aging and increases longevity without increasing cancer. EMBO Mol Med 4(8):691–704. doi:10.1002/emmm.201200245
Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, Yoshimori T, Ohsumi Y, Tokuhisa T, Mizushima N (2004) The role of autophagy during the early neonatal starvation period. Nature 432(7020):1032–1036. doi:10.1038/nature03029
Scott RC, Schuldiner O, Neufeld TP (2004) Role and regulation of starvation-induced autophagy in the Drosophila fat body. Dev Cell 7(2):167–178. doi:10.1016/j.devcel.2004.07.009
Li L, Chen Y, Gibson SB (2012) Starvation-induced autophagy is regulated by mitochondrial reactive oxygen species leading to AMPK activation. Cell Signal 25(1):50–65. doi:10.1016/j.cellsig.2012.09.020
Klionsky DJ, Emr SD (2000) Autophagy as a regulated pathway of cellular degradation. Science 290(5497):1717–1721
Liu Q, Shang F, Guo W, Hobbs M, Valverde P, Reddy V, Taylor A (2004) Regulation of the ubiquitin proteasome pathway in human lens epithelial cells during the cell cycle. Exp Eye Res 78(2):197–205
Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S (2004) Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol 14(10):885–890. doi:10.1016/j.cub.2004.03.059
Cui M, Yu H, Wang J, Gao J, Li J (2013) Chronic caloric restriction and exercise improve metabolic conditions of dietary-induced obese mice in autophagy correlated manner without involving AMPK. J Diabetes Res 2013:852754. doi:10.1155/2013/852754
Minina EA, Sanchez-Vera V, Moschou PN, Suarez MF, Sundberg E, Weih M, Bozhkov PV (2013) Autophagy mediates caloric restriction-induced lifespan extension in Arabidopsis. Aging Cell 12(2):327–329. doi:10.1111/acel.12048
Imai S, Armstrong CM, Kaeberlein M, Guarente L (2000) Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 403(6771):795–800. doi:10.1038/35001622
Sun J, Kale SP, Childress AM, Pinswasdi C, Jazwinski SM (1994) Divergent roles of RAS1 and RAS2 in yeast longevity. J Biol Chem 269(28):18638–18645
Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R (1993) A C. elegans mutant that lives twice as long as wild type. Nature 366(6454):461–464. doi:10.1038/366461a0
Kenyon C (2005) The plasticity of aging: insights from long-lived mutants. Cell 120(4):449–460. doi:10.1016/j.cell.2005.02.002
Toth ML, Sigmond T, Borsos E, Barna J, Erdelyi P, Takacs-Vellai K, Orosz L, Kovacs AL, Csikos G, Sass M, Vellai T (2008) Longevity pathways converge on autophagy genes to regulate life span in Caenorhabditis elegans. Autophagy 4(3):330–338
Keller JN, Huang FF, Markesbery WR (2000) Decreased levels of proteasome activity and proteasome expression in aging spinal cord. Neuroscience 98(1):149–156
Keller JN, Gee J, Ding Q (2002) The proteasome in brain aging. Ageing Res Rev 1(2):279–293
Low P (2011) The role of ubiquitin–proteasome system in ageing. Gen Comp Endocrinol 172(1):39–43. doi:10.1016/j.ygcen.2011.02.005
Necchi D, Lomoio S, Scherini E (2011) Dysfunction of the ubiquitin–proteasome system in the cerebellum of aging Ts65Dn mice. Exp Neurol 232(2):114–118. doi:10.1016/j.expneurol.2011.08.009
Shintani T, Klionsky DJ (2004) Autophagy in health and disease: a double-edged sword. Science 306(5698):990–995. doi:10.1126/science.1099993
Salminen A, Kaarniranta K, Kauppinen A, Ojala J, Haapasalo A, Soininen H, Hiltunen M (2013) Impaired autophagy and APP processing in Alzheimer’s disease: the potential role of Beclin 1 interactome. Prog Neurobiol 106–107:33–54. doi:10.1016/j.pneurobio.2013.06.002
Wang F, Durfee LA, Huibregtse JM (2013) A cotranslational ubiquitination pathway for quality control of misfolded proteins. Mol Cell 50(3):368–378. doi:10.1016/j.molcel.2013.03.009
Schreiber A, Peter M (2013) Substrate recognition in selective autophagy and the ubiquitin–proteasome system. Biochim Biophys Acta. doi:10.1016/j.bbamcr.2013.03.019
Cooney R, Baker J, Brain O, Danis B, Pichulik T, Allan P, Ferguson DJ, Campbell BJ, Jewell D, Simmons A (2010) NOD2 stimulation induces autophagy in dendritic cells influencing bacterial handling and antigen presentation. Nat Med 16(1):90–97. doi:10.1038/nm.2069
Shoji-Kawata S, Sumpter R, Leveno M, Campbell GR, Zou Z, Kinch L, Wilkins AD, Sun Q, Pallauf K, MacDuff D, Huerta C, Virgin HW, Helms JB, Eerland R, Tooze SA, Xavier R, Lenschow DJ, Yamamoto A, King D, Lichtarge O, Grishin NV, Spector SA, Kaloyanova DV, Levine B (2013) Identification of a candidate therapeutic autophagy-inducing peptide. Nature 494(7436):201–206. doi:10.1038/nature11866
Li X, Fan Z (2010) The epidermal growth factor receptor antibody cetuximab induces autophagy in cancer cells by downregulating HIF-1alpha and Bcl-2 and activating the beclin 1/hVps34 complex. Cancer Res 70(14):5942–5952. doi:10.1158/0008-5472.CAN-10-0157
Park KJ, Lee SH, Kim TI, Lee HW, Lee CH, Kim EH, Jang JY, Choi KS, Kwon MH, Kim YS (2007) A human scFv antibody against TRAIL receptor 2 induces autophagic cell death in both TRAIL-sensitive and TRAIL-resistant cancer cells. Cancer Res 67(15):7327–7334. doi:10.1158/0008-5472.CAN-06-4766
Kawaguchi Y, Kovacs JJ, McLaurin A, Vance JM, Ito A, Yao TP (2003) The deacetylase HDAC6 regulates aggresome formation and cell viability in response to misfolded protein stress. Cell 115(6):727–738
Pandey UB, Nie Z, Batlevi Y, McCray BA, Ritson GP, Nedelsky NB, Schwartz SL, DiProspero NA, Knight MA, Schuldiner O, Padmanabhan R, Hild M, Berry DL, Garza D, Hubbert CC, Yao TP, Baehrecke EH, Taylor JP (2007) HDAC6 rescues neurodegeneration and provides an essential link between autophagy and the UPS. Nature 447(7146):859–863. doi:10.1038/nature05853
He P, Peng Z, Luo Y, Wang L, Yu P, Deng W, An Y, Shi T, Ma D (2009) High-throughput functional screening for autophagy-related genes and identification of TM9SF1 as an autophagosome-inducing gene. Autophagy 5(1):52–60
Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T (2000) LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 19(21):5720–5728. doi:10.1093/emboj/19.21.5720
Chan EY, Kir S, Tooze SA (2007) siRNA screening of the kinome identifies ULK1 as a multidomain modulator of autophagy. J Biol Chem 282(35):25464–25474. doi:10.1074/jbc.M703663200
Arsham AM, Neufeld TP (2009) A genetic screen in Drosophila reveals novel cytoprotective functions of the autophagy-lysosome pathway. PLoS One 4(6):e6068. doi:10.1371/journal.pone.0006068
Takeuchi H, Kondo Y, Fujiwara K, Kanzawa T, Aoki H, Mills GB, Kondo S (2005) Synergistic augmentation of rapamycin-induced autophagy in malignant glioma cells by phosphatidylinositol 3-kinase/protein kinase B inhibitors. Cancer Res 65(8):3336–3346. doi:10.1158/0008-5472.CAN-04-3640
Ravikumar B, Duden R, Rubinsztein DC (2002) Aggregate-prone proteins with polyglutamine and polyalanine expansions are degraded by autophagy. Hum Mol Genet 11(9):1107–1117
Ravikumar B, Vacher C, Berger Z, Davies JE, Luo S, Oroz LG, Scaravilli F, Easton DF, Duden R, O’Kane CJ, Rubinsztein DC (2004) Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet 36(6):585–595. doi:10.1038/ng1362
Martinet W, Verheye S, De Meyer GR (2007) Everolimus-induced mTOR inhibition selectively depletes macrophages in atherosclerotic plaques by autophagy. Autophagy 3(3):241–244
Crazzolara R, Bradstock KF, Bendall LJ (2009) RAD001 (Everolimus) induces autophagy in acute lymphoblastic leukemia. Autophagy 5(5):727–728
Burns J, Yokota T, Ashihara H, Lean ME, Crozier A (2002) Plant foods and herbal sources of resveratrol. J Agric Food Chem 50(11):3337–3340
Finkel T, Deng CX, Mostoslavsky R (2009) Recent progress in the biology and physiology of sirtuins. Nature 460(7255):587–591. doi:10.1038/nature08197
Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA (2006) Resveratrol improves health and survival of mice on a high-calorie diet. Nature 444(7117):337–342. doi:10.1038/nature05354
Morselli E, Maiuri MC, Markaki M, Megalou E, Pasparaki A, Palikaras K, Criollo A, Galluzzi L, Malik SA, Vitale I, Michaud M, Madeo F, Tavernarakis N, Kroemer G (2010) Caloric restriction and resveratrol promote longevity through the Sirtuin-1-dependent induction of autophagy. Cell Death Dis 1:e10. doi:10.1038/cddis.2009.8
Morselli E, Maiuri MC, Markaki M, Megalou E, Pasparaki A, Palikaras K, Criollo A, Galluzzi L, Malik SA, Vitale I, Michaud M, Madeo F, Tavernarakis N, Kroemer G (2010) The life span-prolonging effect of sirtuin-1 is mediated by autophagy. Autophagy 6(1):186–188
Bursch W, Ellinger A, Kienzl H, Torok L, Pandey S, Sikorska M, Walker R, Hermann RS (1996) Active cell death induced by the anti-estrogens tamoxifen and ICI 164 384 in human mammary carcinoma cells (MCF-7) in culture: the role of autophagy. Carcinogenesis 17(8):1595–1607
Cho KS, Yoon YH, Choi JA, Lee SJ, Koh JY (2012) Induction of autophagy and cell death by tamoxifen in cultured retinal pigment epithelial and photoreceptor cells. Invest Ophthalmol Vis Sci 53(9):5344–5353. doi:10.1167/iovs.12-9827
Eisenberg T, Knauer H, Schauer A, Buttner S, Ruckenstuhl C, Carmona-Gutierrez D, Ring J, Schroeder S, Magnes C, Antonacci L, Fussi H, Deszcz L, Hartl R, Schraml E, Criollo A, Megalou E, Weiskopf D, Laun P, Heeren G, Breitenbach M, Grubeck-Loebenstein B, Herker E, Fahrenkrog B, Frohlich KU, Sinner F, Tavernarakis N, Minois N, Kroemer G, Madeo F (2009) Induction of autophagy by spermidine promotes longevity. Nat Cell Biol 11(11):1305–1314. doi:10.1038/ncb1975
Acknowledgments
This work was supported by the Department of Biotechnology, Government of India. AM was supported by Ramalinganswami Fellowship and Innovative Young Biotechnologist Award (IYBA) scheme from the Department of Biotechnology, Government of India. The authors would like to thank Mr. Bharat Pareek and Mr. Rahul Sathya Babu for their technical assistance and the entire lab management during the manuscript preparation. We apologize to various authors whose work could not be included due to space limitations.
Conflict of Interest
The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chhangani, D., Chinchwadkar, S. & Mishra, A. Autophagy Coupling Interplay: Can Improve Cellular Repair and Aging?. Mol Neurobiol 49, 1270–1281 (2014). https://doi.org/10.1007/s12035-013-8599-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-013-8599-z