Abstract
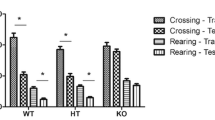
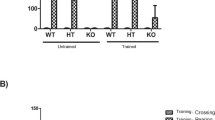
Congenital muscular dystrophies present mutated gene in the LARGE mice model and it is characterized by an abnormal glycosylation of α-dystroglycan (α-DG), strongly implicated as having a causative role in the development of central nervous system abnormalities such as cognitive impairment seen in patients. However, the pathophysiology of the brain involvement remains unclear. Therefore, the objective of this study is to evaluate the oxidative damage and energetic metabolism in the brain tissue as well as cognitive involvement in the LARGE(myd) mice model of muscular dystrophy. With this aim, we used adult homozygous, heterozygous, and wild-type mice that were divided into two groups: behavior and biochemical analyses. In summary, it was observed that homozygous mice presented impairment to the habituation and avoidance memory tasks; low levels of brain-derived neurotrophic factor (BDNF) in the prefrontal cortex, hippocampus, cortex and cerebellum; increased lipid peroxidation in the prefrontal cortex, hippocampus, striatum, and cerebellum; an increase of protein peroxidation in the prefrontal cortex, hippocampus, striatum, cerebellum, and cortex; a decrease of complex I activity in the prefrontal cortex and cerebellum; a decrease of complex II activity in the prefrontal cortex and cerebellum; a decrease of complex IV activity in the prefrontal cortex and cerebellum; an increase in the cortex; and an increase of creatine kinase activity in the striatum and cerebellum. This study shows the first evidence that abnormal glycosylation of α-DG may be affecting BDNF levels, oxidative particles, and energetic metabolism thus contributing to the memory storage and restoring process.




Similar content being viewed by others
References
van der Knaap MS, Smit LM, Barth PG, Catsman-Berrevoets CE, Brouwer OF, Begeer JH (2007) Magnetic resonance imaging in classification of congenital muscular dystrophies with brain abnormalities. Ann Neurol 42:50–59
Parano E, Pavone L, Fiumara A, Falsaperla R, Trifiletti RR, Dobyns WB (1995) Congenital muscular dystrophies: clinical review and proposed classification. Pediatr Neurol 13:97–103
Jimenez-Mallebrera C, Brown SC, Sewry CA, Muntoni F (2005) Congenital muscular dystrophy: molecular and cellular aspects. Cell Mol Life Sci 62:809–823
Lane PW, Beamer TC, Myers DD (1976) Myodystrophy, a new myopathy on chromosome 8 of the mouse. J Hered 67:135–138
Rayburn HB, Peterson AC (1978) Naked axons in myodystrophic mice. Brain Res 146:380–384
Michele DE, Barresi R, Kanagawa M (2002) Post-translational disruption of dystroglycan–ligand interactions in congenital muscular dystrophies. Nature 418:417–421
Holzfeind PJ, Grewal PK, Reitsamer HA (2002) Skeletal, cardiac and tongue muscle pathology, defective retinal transmission, and neuronal migration defects in the Large(myd) mouse defines a natural model for glycosylation-deficient muscle–eye–brain disorders. Hum Mol Genet 11:2673–2687
Longman C, Brockington M, Torelli S, Jimenez-Mallebrera C, Kennedy C, Khalil N, Feng L, Saran RK, Voit T, Merlini L, Sewry CA, Brown SC, Muntoni F (2003) Mutations in the human LARGE gene cause MDC1D, a novel form of congenital muscular dystrophy with severe mental retardation and abnormal glycosylation of alpha-dystroglycan. Hum Mol Genet 12:2853–2861
Yoshida A, Kobayashi K, Manya H, Taniguchi K, Kano H, Mizuno M, Inazu T, Mitsuhashi H, Takahashi S, Takeuchi M, Herrmann R, Straub V, Talim B, Voit T, Topaloglu H, Toda T, Endo T (2001) Muscular and neuronal migration disorder caused by mutations in a glycosyltransferase, POMGnT1. Dev Cell 1:717–724
Li J, Yu M, Feng G, Hu H, Li X (2001) Breaches of the pial basement membrane are associated with defective dentate gyrus development in mouse models of congenital muscular dystrophies. Neurosci Lett 505:19–24
Bramham CR, Messaoudi E (2005) BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog Neurobiol 76:99–125
Francia N, Cirulli F, Chiarotti F, Antonelli A, Aloe L, Alleva E (2006) Spatial memory deficits in middle-aged mice correlate with lower exploratory activity and a subordinate status: role of hippocampal neurotrophins. Eur J Neurosci 23:711–728
Floyd RA (1999) Antioxidants, oxidative stress, and degenerative neurological disorders. Proc Soc Exp Biol Med 222:236–245
Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donald-son D, Goto J, O’Regan JP, Deng HX, Rahmani Z, Krizus A, McKenna-Yasek D, Cayabyab A, Gaston SM, Berger R, Tanzi RE, Halperin JJ, Herzfeldt B, Bergh RV, Hung W-Y, Bird T, Deng G, Mulder DW, Smyth C, Laing NG, Soriano E, Pericak-Vance MA, Haines J, Rouleau GA, Gusella JSH, Horvitz R, Brown RH Jr (1993) Mutations in Cu/Zn-superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 362:59–62
Durany N, Munch G, Michel T, Riederer P (1999) Investigations on oxidative stress and therapeutical implications in dementia. Eur Arch Psychiatr Clin Neurosci 249:68–73
Abraham S, Soundararajan CC, Vivekanandhan S, Behari M (2005) Erythrocyte antioxidant enzymes in Parkinson’s disease. Indian J Med Res 121:111–115
Lucca G, Comim CM, Valvassori SS, Reus GZ, Vuolo F, Petronilho F, Gavioli EC, Dal-Pizzol F, Quevedo J (2009) Increased oxidative stress in submitochondrial particles into the brain of rats submitted to the chronic mild stress paradigm. J Psychiatr Res 43:864–869
Lucca G, Comim CM, Valvassori SS, Reus GZ, Vuolo F, Petronilho F, Dal-Pizzol F, Gavioli EC, Quevedo J (2009) Effects of chronic mild stress on the oxidative parameters in the rat brain. Neurochem Int 54:358–362
Comim CM, Cassol-Jr OJ, Constantino LC, Constantino LS, Petronilho F, Tuon L, Vainzof M, Dal-Pizzol F, Quevedo J (2009) Oxidative variables and antioxidant enzymes activities in the mdx mouse brain. Neurochem Int 55:802–805
Heales SJ, Bolaños JP, Stewart VC, Brookes PS, Land JM, Clark J (1999) Nitric oxide, mitochondria and neurological disease. Biochim Biophys Acta 1410:215–228
Wyss M, Kaddurah-Daouk R (2003) Creatine and creatinine metabolism. Physiol Rev 3(110):8–1182
Browning CA, Grewal PK, Moore CJ, Hewitt JE (2005) A rapid PCR method for genotyping the Large(myd) mouse, a model of glycosylation-deficient congenital muscular dystrophy. Neuromuscul Disord 15:331–335
Esterbauer H, Cheeseman KH (1990) Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol 186:407–421
Levine RL, Williams JA, Stadtman ER, Shacter E (1994) Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol 233:346–357
Cassina A, Radi R (1996) Differential inhibitory action of nitric oxide and peroxynitrite on mitochondrial electron transport. Arch Biochem Biophys 328:309–316
Fischer JC, Ruitenbeek W, Berden JA, Trijbels JM, Veerkamp JH, Stadhouders AM, Sengers RC, Janssen AJ (1985) Differential investigation of the capacity of succinate oxidation in human skeletal muscle. Clin Chim Acta 153:23–36
Miro O, Cardellach F, Barrientos A, Casademont J, Rotig A, Rustin P (1998) Cytochrome c oxidase assay in minute amounts of human skeletal muscle using single wavelength spectrophotometers. J Neurosci Meth 80:107–111
Hughes BP (1962) A method for the estimation of serum creatine kinase and its use in comparing creatine kinase and aldolase activity in normal and pathological sera. Clin Chim Acta 7:597–603
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 1:265–275
Zaccaria ML, di Tommaso F, Brancaccio A, Paggi P, Petrucci TC (2001) Dystroglycan distribution in adult mouse brain: a light and electron microscopy study. Neuroscience 104:311–324
Anderson JL, Head SI, Rae C, Morley JW (2002) Brain function in Duchenne muscular dystrophy. Brain 125:4–13
Huang CC, Kuo HC (2005) Myotonic dystrophies. Chang Gung Med J 28:517–526
Tuon L, Comim CM, Fraga DB, Scaini G, Rezin GT, Baptista BR, Streck EL, Vainzof M, Quevedo J (2010) Mitochondrial respiratory chain and creatine kinase activities in mdx mouse brain. Muscle Nerve 41:257–260
Halliwell B, Gutteridge JM (1997) Lipid peroxidation in brain homogenates: the role of iron and hydroxyl radicals. J Neurochem 69:1330–1331
Mancuso C, Scapagini G, Currò D, Giuffrida Stella AM, De Marco C, Butterfield DA, Calabrese V (2007) Mitochondrial dysfunction, free radical generation and cellular stress response in neurodegenerative disorders. Front Biosci 12:1107–1123
Navarro A, Boveris A (2007) The mitochondrial energy transduction system and the aging process. Am J Physiol Cell Physiol 292:C670–C 686
Lipton P, Whittingham TS (1982) Reduced ATP concentration as a basis for synaptic transmission failure during hypoxia in the in vitro guinea pig hippocampus. J Physiol 325:51–65
Green DE, Fry M (1980) On reagents that convert cytochrome oxidase from an inactive to an active coupling state. Proc Nat l Acad Sci USA 77(1951–19):55
Brustovetsky N, Brustovetsky T, Dubinsky JM (2001) On the mechanisms of neuroprotection by creatine and phosphocreatine. J Neuro Chem 76:425–434
Acknowledgments
This research was supported by grants from CNPq (CMC, JQ, FD-P, and ELS), FAPESC (JQ, FD-P and ELS), and UNESC (JQ, FD-P, and ELS). ELS, FDP, and JQ are CNPq research fellows. CMC is a holder of a CNPq studentship.
Conflict of interest
None of the authors or funding sources has conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Comim, C.M., Mendonça, B.P., Dominguini, D. et al. Central Nervous System Involvement in the Animal Model of Myodystrophy. Mol Neurobiol 48, 71–77 (2013). https://doi.org/10.1007/s12035-013-8415-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-013-8415-9